 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 26(1); 2024 > Article |
|
Abstract
Fig. 1.
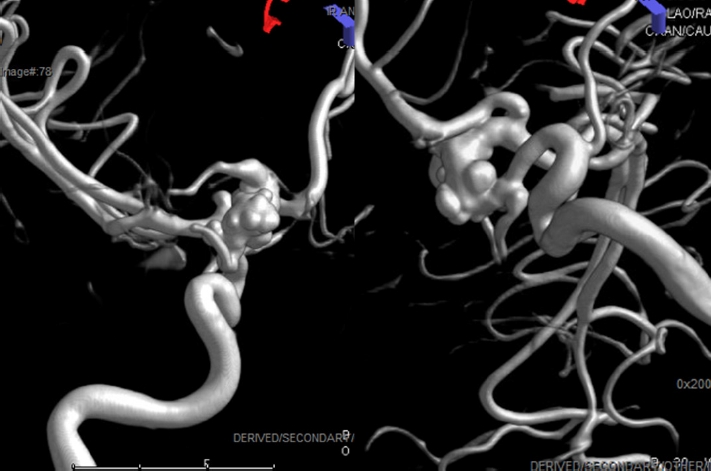
Fig. 2.

Fig. 3.

Fig. 4.
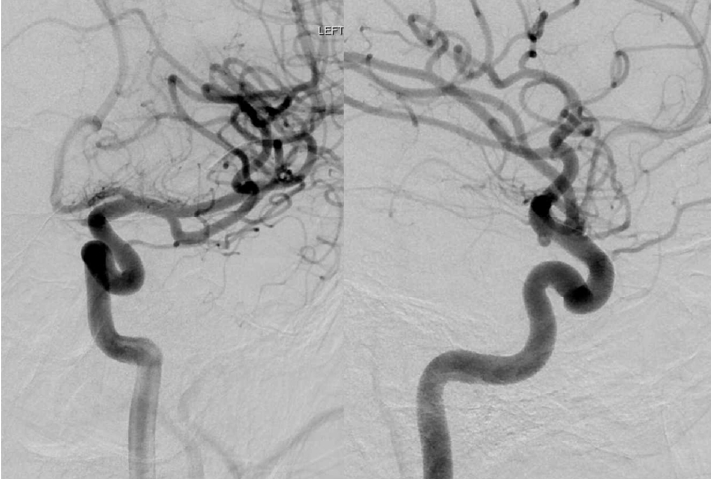
Table 1.
| Author and year | Ref. | N. of PEDs | Aneurysm locations | Age (years) | Sex | Aneurysm features |
|---|---|---|---|---|---|---|
| Kumaria et al. 2021 | [22] | 1 | ICA | 8 | 1 male | Serpiginous |
| Colby et al. 2018 | [10] | 1 | MCA | 0.75 | 1 female | Saccular |
| Mohammad et al. 2017 | [26] | 1 | Basilar | 15 | 1 male | Partially thrombosed aneurysm |
| Vachhani et al. 2016 | [35] | 1 | A2 | 12 | 1 female | Fusiform |
| Vargas et al. 2016 | [36] | 1 | A2 | 8 | 1 male | Saccular |
| 2 | Basilar, ICA | 9 | 1 male | Fusiform | ||
| Kan et al. 2016 | [19] | 1 | Basilar | 16 | 1 male | Fusiform |
| Takemoto et al. 2014 | [32] | 2 | ICA, Acom | N/A | N/A | N/A |
| Burrows et al. 2013 | [6] | 1 | M2 | 15 | 1 male | Fusiform |
| Saraf et al. 2012 | [30] | 1 | Basilar | N/A | N/A | Partially thrombosed aneurysm |
| Crowley et al. 2009 | [11] | 1 | Basilar | 1.8 | 1 male | Fusiform |
| Ares et al. 2019 | [4] | 1 | Basilar | 2 | 1 male | Infectious, Sub occlusive thrombosed aneurysm |
| Tonetti et al. 2021 | [33] | 1 | A2 | 15 | 1 male | Fusiform, Partially thrombosed |
| Cherian et al. 2021 | [7] | 46 | N/A | Range (3-21) | 27 male | N/A |
| 12 female | ||||||
| Samples et al. 2021 | [29] | 1 | M1 | 10 | 1 female | Ruptured, Infectious |
| Cunegatto-Braga 2018 | [12] | 1 | PCA | 4 | 1 male | Ruptured, Dissecting |
| Ghali et al. 2018 | [15] | 1 | ICA | 13 | 1 male | N/A |
| 1 | ACA | 14 | 1 male | Traumatic | ||
| 1 | Basilar | 1.16 | 1 male | Dissecting | ||
| Sastry et al. 2018 | [31] | 1 | Basilar | 13 | 1 male | Iatrogenic |
| Navarro et al. 2015 | [27] | 1 | Pcom | 4 | Male | Saccular |
| 2 | Vertebrobasilar, ICA | 11 | 1 female | Saccular | ||
| Jia et al. 2020 | [17] | 1 | Vertebrobasilar | N/A | 1 female | Dissecting |
| Barburoglu and Arat 2017 | [5] | 7 | (4) ICA, (1) A2, (1) M1, (1) V4 | Range (3-16) | 6 males | N/A |
| 1 female | ||||||
| Ikeda et al. 2015 | [16] | 1 | M2 | 12 | 1 female | Fusiform |
| Appelboom et al. 2010 | [3] | 1 | ICA | 10 | 1 female | Infectious |
| Lin et al. 2016 | [24] | 1 | A2 | 13 | 1 male | Dissecting |
| 3 | M2 | 16 | 1 male | Fusiform | ||
| De Barros et al. 2011 | [13] | 2 | Basilar | 12 | 1 female | Dissecting |
| 2 | Basilar | 5 | 1 female | Dissecting | ||
| 4 | Vertebrobasilar | 7 | 1 male | Dissecting | ||
| Cinar et al. 2013 | [8] | 1 | ICA | 8 | 1 female | Saccular |
| Zarzecka et al. 2014 | [37] | 2 | V4 | 15 | 1 male | Fusiform |
| Abla et al. 2014 | [1] | 1 | ICA | 10 | 1 male | Fusiform |
| Trivelato et al. 2017 | [34] | 1 | ICA | 9 | 1 female | Traumatic |
| Cobb et al. 2017 | [9] | 1 | ICA | 13 | 1 female | Iatrogenic |
| Lubicz et al. 2010 | [25] | 1 | Basilar | 17 | 1 female | Fusiform |
| 1 | ICA | 10 | 1 female | Saccular |
Ref., reference number; N. of PEDs, number of pediatric patients; ICA, internal cerebral artery; MCA, middle cerebral artery; A2, second segment of the anterior cerebral artery; Acom, anterior communicating artery; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery; PCA, posterior cerebral artery; ACA, anterior cerebral artery; Pcom, posterior communicating artery; V4, fourth segment of the vertebral artery
REFERENCES
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,316 View
- 26 Download
- ORCID iDs
-
Akram M Eraky

https://orcid.org/0000-0003-4219-3659 - Related articles
-
Seven Intracranial Aneurysms in One Patient: Treatment and Review of Literature2015 June;17(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



