 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 17(1); 2015 > Article |
|
Abstract
Cavernous malformations (CMs), characterized by the presence of a hemosiderin rim and intralesional hemorrhage, are relatively common intracranial vascular malformations. Extralesional hemorrhages arising from CMs are seen in a minority of cases, but most of them show typical CM findings on magnetic resonance imaging. Here, the authors report two cases of pathologically confirmed CM presenting with unusual and large intracerebral hemorrhages, which were not surrounded by the typical hemosiderin rim. CMs presenting with large intracerebral hemorrhage should be considered in the differential diagnosis of massive intracerebral hemorrhages.
Cavernous malformations (CMs) are congenital vascular anomalies of the central nervous system, and the second most frequent intracranial vascular malformation responsible for hemorrhages.9) While most hemorrhages from CMs are characterized by asymptomatic intralesional microhemorrhage,4) the less common extralesional hemorrhages are usually symptomatic. CMs presenting with extralesional hemorrhages are usually defined in magnetic resonance imaging (MRI) by the presence of acute or subacute blood outside the hemosiderin rim.12) We report here two cases of a CM presenting with unusual and large intracerebral hemorrhages without a surrounding typical hemosiderin rim.
A 31-year-old female was brought to the emergency room following a generalized tonic-clonic seizure that lasted 5 minutes. Her mental status was alert, and the neurologic examination was unremarkable. Brain computed tomography (CT) showed a 4.8-cm-diameter hyperdense mass with perilesional edema in the right frontal lobe (Fig. 1A). She underwent brain MRI, which showed an aciniform, 4.8-cm-sized intraaxial mass, combined with severe perilesional edema and midline shifting, in the right frontal lobe. The mass demonstrated hyperintensity on T1- and T2-weighted MR images and subtle enhancement after intravenous gadolinium injection, which suggested intratumoral bleeding (Fig. 1B-D). She underwent craniotomy and removal of the mass. In intraoperative findings, the mass was composed of mostly multiaged hematomas without capsule, and a small subcortical mass filled with old hematoma and necrotic tissue. In histopathological findings, a small subcortical mass consisted of dilated, thin-walled capillaries having an endothelial lining consisting of one layer of cells, and a variable layer of fibrous adventitia without intervening neural tissue (Fig. 1E, F). These findings were consistent with a cerebral cavernous malformation. Following surgery, the patient's clinical course was uneventful.
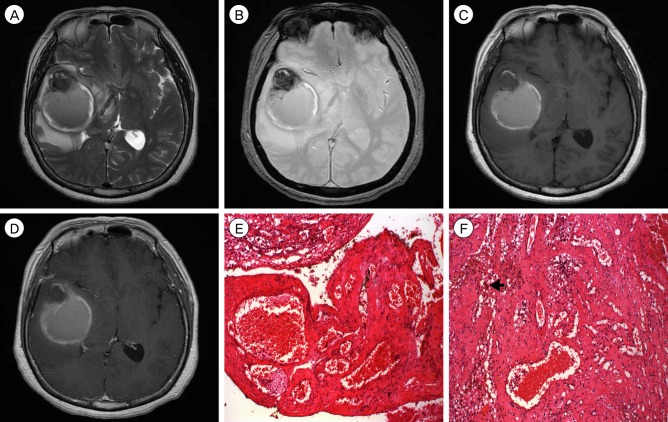
A 59-year-old female presented with severe headache, nausea, and vomiting beginning a few days previously. Neurological examination was unremarkable. MRI showed a 6-cm-diameter hematoma in the right temporal lobe. The hematoma showed a mixed signal of subacute and acute hematoma, and had caused midline shifting. There was no enhancement of the lesion after intravenous gadolinium injection (Fig. 2A-D). No abnormal vascular was observed on cerebral angiography. She underwent craniotomy and removal of hematomas. Intraoperative findings revealed old liquefied hematoma mixed with acute hemorrhage, and a small amount of adjacent abnormal brain tissue. All abnormal elements were removed and biopsied. Histopathological findings demonstrated interconnecting thin membranous vascular channels without muscle layer and many hemosiderin-laden macrophages (Fig. 2E, F). Three months after surgery, the patient was uneventful, and a follow-up MRI showed unremarkable findings.
CMs are composed of sinusoidal vascular channels lined by a single layer of endothelium. Vascular channels contain thrombi of varying ages, separated by fibrotic tissue containing foci of calcification and hemosiderin deposition. CMs are characterized by a lack of intervening brain parenchyma, and the surrounding parenchyma exhibits evidence of previous microhemorrhages, namely hemosiderin deposition, and hemosiderin-filled macrophages.9) Repeated small-volume hemorrhages within the lesion cause a progressive increase in the size of the CM over time. Kondziolka et al.9) reported prospective hemorrhage and rehemorrhage rates of 2.% to 5% per year.
CMs are angiographically occult vascular malformations,9) usually diagnosed by typical MRI characteristics. Zabramski et al.12) described a classification of CMs based on their characteristic appearance in MRI. Most lesion types, except type IV CM, commonly demonstrate a hypointense hemosiderin rim in T2-weighted images. Type IV CM is poorly seen or not visualized at all in both T1- and T2- weighted images, and it is known to never be symptomatic and very low risk for hemorrhage.
CMs rarely produce extralesional hemorrhages into the surrounding brain tissue. The extralesional hemorrhage usually consists only of a focal area of subacute bleeding extending outside the capsule of the lesion.2)3) In the MRI classification of Zabramski et al.,12) type 1A is designated for CMs accompanied by "overt" extralesional hemorrhages extending outside the lesion capsule. Only a few CMs with massive hemorrhage have been reported in the literature. Corapcioglu et al.3) and Chicani et al.2) reported giant intracranial CMs presenting with massive hemorrhage. However, the former described an intralesional hemorrhage, while the latter did not show whether or not the hemorrhage was an extralesional hemorrhage because brain MRI was not performed. Kim et al.8) recently reported on a CM presenting with massive extralesional hemorrhage, and brain MRI showed the classic "popcorn ball" configuration of a CM combined with acute and subacute massive hemorrhage.
The mechanism of massive extralesional hemorrhage is still unknown. However, based on the reported cases, several hypotheses can be established. First, the possibility of the coexistence of a CM and other occult vascular anomaly can be considered. Typically, CMs cause small intralesional hemorrhages because of the absence of a connection to the high-flow vascular system. However, CMs combined with occult arteriovenous malformations or venous anomalies have been reported,1)6) and coexisting vascular anomaly causing massive hemorrhage is possible. Second, low resistance of hemosiderin rim and surrounding brain tissue may be influencing factors of extralesional hemorrhage from a CM. Schmitt et al.10) reported subdural hematoma from a CM and suggested that the proximity of a CM to the cortical surface likely offered a path of low resistance and predisposed the patient to a large extralesional hemorrhage. In addition, comparable rates of rebleeding have been reported after incomplete resection of CMs, presumably because of interruption of the lesion capsule.7)11) Based on our intraoperative findings of hematomas of various age, repeated hemorrhages should influence the resistance of hemosiderin rim and gliosis.
Distinguishing extralesional hemorrhage of a CM from other causes of intracerebral hemorrhage such as malignant tumor or vascular lesion is not easy.5) In the presented case, CMs were not included as a preoperative presumptive diagnosis either, because of the rarity of CM and unusual imaging findings.
References
1. Awad IA, Robinson JR Jr, Mohanty S, Estes ML. Mixed vascular malformations of the brain: clinical and pathogenetic considerations. Neurosurgery. 1993 8;33(2):179-188;


2. Chicani CF, Miller NR, Tamargo RJ. Giant cavernous malformation of the occipital lobe. J Neuroophthalmol. 2003 6;23(2):151-153;


3. Corapçioğlu F, Akansel G, Gönüllü E, Yildiz K, Etuş V. Fatal giant pediatric intracranial cavernous angioma. Turk J Pediatr. 2006 Jan-Mar;48(1):89-92;

4. Flemming KD, Link MJ, Christianson TJ, Brown RD Jr. Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. 2012 2;78(9):632-636;


5. Furlan AJ, Whisnant JP, Elveback LR. The decreasing incidence of primary intracerebral hemorrhage: a population study. Ann Neurol. 1979 4;5(4):367-373;


6. Garner TB, Del Curling, Kelly DL Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg. 1991 11;75(5):715-722;


7. Jain KK, Robertson E. Recurrence of an excised cavernous hemangioma in the opposite cerebral hemisphere. Case report. J Neurosurg. 1970 10;33(4):453-456;


8. Kim YS, Lee JI, Choi CH, Ko JK. Massive intracerebral hemorrhage caused by a cavernous malformation. J Korean Neurosurg Soc. 2012 1;51(1):37-39;




9. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995 11;83(5):820-824;


10. Schmitt AJ, Mitha AP, Germain R, Eschbacher J, Spetzler RF. Subdural hematoma from a cavernous malformation. World Neurosurg. 2014 Sep-Oct;82(3-4):535.e1-3.

Fig. 1
(A) Axial computed tomography scan shows a hemorrhagic intraaxial lesion in the right frontal lobe. (B-D) Magnetic resonance imaging demonstrates mixed intensity on T1- and T2-weighted images and slight enhancement after intravenous gadolinium injection. (E,F) Histopathologic findings reveal dilated, thin-walled capillaries having an endothelial lining consisting of one layer of cells, and a variable layer of fibrous adventitia without intervening neural tissue. (Hematoxylin and Eosin Stain ×40,×100)

Fig. 2
(A-D) Magnetic resonance imaging shows a mixed signal of acute and subacute hematoma, and no enhanced lesion after intravenous gadolinium injection. (E, F) Histopathological findings demonstrate interconnecting thin membranous vascular channels without muscle layer and many hemosiderin-laden macrophages (arrowhead). (Hematoxylin and Eosin Stain ×40,×100)
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 2,878 View
- 19 Download
- Related articles
-
Treatment of Cavernous Angioma Presenting with Epilepsy.2001 September;3(2)
Acute Expansion of Hematoma in Hypertensive Intracerebral Hemorrhage.2001 September;3(2)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



