Endovascular Treatments for Ruptured Intracranial Vertebral Artery Dissecting Aneurysms: Experience in 16 Patients
Article information
Abstract
Objective
Intracranial vertebral artery dissecting aneurysms are rare lesions that are considered an important cause of spontaneous subarachnoid hemorrhage. We report our decade-long experience in treating ruptured intracranial vertebral artery dissecting aneurysms.
Materials and Methods
This retrospective single-center study included 21 consecutive patients between February 2005 and March 2015. Their clinical features included radiologic finding at the initial examination, treatment modality, functional outcome at the last follow-up, mortality, and radiologic outcome at more than 6 months after the initial treatment.
Results
All 16 aneurysms were treated endovascularly; aneurysm trapping was performed in 9 patients and vascular reconstruction was performed in 7 patients. For 6 aneurysms involving the posterior inferior cerebellar artery (PICA), the modalities of treatment were aneurysm trapping in 3 patients and vascular reconstruction in 3 patients. The mean duration of follow-up was 29 months (range, 6–70 months). Five patients expired, indicating a mortality rate of 31%. In surviving patients, the unfavorable outcome rate (modified Rankin Scale [mRS] > 2) was 36%. The overall mean mRS for survivors was 1.8. Angiographic follow-up in 11 survivors at 13 months, (range, 6–46 months) revealed recanalization of the aneurysm in one patient.
Conclusions
Ruptured intracranial vertebral artery dissecting aneurysm is associated with poor functional outcome and high mortality. More immediate treatments are needed due to the high rebleeding rate in this disease condition. Endovascular treatment may be a useful option for ruptured intracranial vertebral artery dissecting aneurysms.
INTRODUCTION
The incidence of intracranial vertebral artery (VA) dissecting aneurysms is 0.001–0.0015% in the general population,17)20) and intracranial VA dissecting aneurysms are lesions that are associated with significant morbidity and mortality.1)11)22)
There are two major types of VA dissection; the ischemic type and the hemorrhagic type. The ischemic type presents with vertebrobasilar insufficiency or posterior circulation infarction due to arterial occlusion and thromboembolism.12) A relatively benign natural history has been reported in cases with ischemic symptoms.8)25) The hemorrhagic type presents with subarachnoid hemorrhage (SAH) due to rupture of an intracranial VA dissecting aneurysm. The hemorrhagic type is comparatively less common than the ischemic type. VA dissecting aneurysms are an important cause of spontaneous SAH, accounting for 3–7%18) of all cases of SAH. Previous studies have reported a high incidence of recurrent bleeding and a high mortality rate in patients with hemorrhagic VA dissecting aneurysms.1)11)22) These reports indicated that very early treatment for a ruptured VA dissecting aneurysm is required to prevent fatal recurrent bleeding.
Additionally, surgical treatment and endovascular treatment of VA dissecting aneurysms have proven successful. Endovascular treatment has been the main treatment option for a VA dissecting aneurysm. In particular, endovascular treatment can achieve not only occlusion of the parent artery but also allow reconstruction of the particular artery. In cases involving the posterior inferior cerebellar artery (PICA), aneurysm trapping has been recommended after occipital artery-PICA bypass surgery.6) However, optimal treatment options for VA dissecting aneurysms are still controversial.
Here, we report our experiences with endovascular treatments for ruptured intracranial VA dissecting aneurysms during a 10-year period. The purpose of this study was to evaluate the safety and efficiency of endovascular treatment for ruptured intracranial VA dissecting aneurysms.
MATERIALS AND METHODS
We conducted a retrospective review of radiologic findings in patients with intracranial VA dissecting aneurysms between February 2005 and March 2015 at our institution. Of these patients, 33 had been diagnosed with intracranial VA dissecting aneurysms and 21 had been diagnosed with ruptured intracranial VA dissecting aneurysms confirmed by either computed tomography (CT) scan and contrast-enhanced three-dimensional CT angiography or magnetic resonance (MR) imaging and angiography. If the diagnosis was uncertain, we performed digital subtraction angiography (DSA). Twelve patients with unruptured aneurysms and observational cases were excluded, and cases of only dissecting aneurysms involving the VA as an intracranial lesion were included. The clinical features at pretreatment were graded according to the World Federation of Neurological Surgeons (WFNS) scale and the presence or absence of recurrent bleeding. Dissecting aneurysms were diagnosed if one or both of the following conditions were met: 1) Angiographic feature of aneurysms was a ‘pear and/or string sign,’ and 2) MR or CT confirmed a false lumen involving the VA. Angiograms were assessed for the maximal size, shape, and location of the aneurysm. The location of each aneurysm was classified into the following 4 categories: 1) proximal to the PICA, 2) involving the PICA, 3) distal to the PICA, and 4) involving the anterior spinal artery.
We immediately treated 21 patients who had ruptured VA dissecting aneurysms with endovascular surgery with or without a stent. All patients were treated within 12 hours after onset under general anesthesia. We chose endovascular surgery for trapping the VA at the lesion site or for preservation of the parent artery and the PICA. After the standard bilateral femoral puncture, bilateral 6 F introducer sheaths or single 6 F introducer sheath was inserted and bilateral 6 F guiding catheters or single 6 F guiding catheter was placed in the proximal VA. Systemic heparinization was not performed. For aneurysm trapping, Guglielmi detachable coils were delivered to the distal portion of the dissection site or to the proximal portion of the aneurysm. In the case of preservation of the parent artery and the PICA, single stent-assisted coil embolization or double overlapping stent-assisted coil embolization was performed and dual antiplatelet medications were administered post-operation. After the procedure, immediate post-operative scans were obtained and reviewed.
The safety of treatment was evaluated based on the incidence of procedure-related complications including rupture of the aneurysm and a thromboembolic or a hemorrhagic event during the procedure. Follow-up clinical evaluations were performed according to the modified Rankin Scale (mRS), with an unfavorable outcome defined as mRS > 2. Follow-up angiography, MR angiography or DSA was performed at 6 months after the operation.
Five patients were excluded from this study due to loss to follow-up and insufficiency of clinical data.
RESULTS
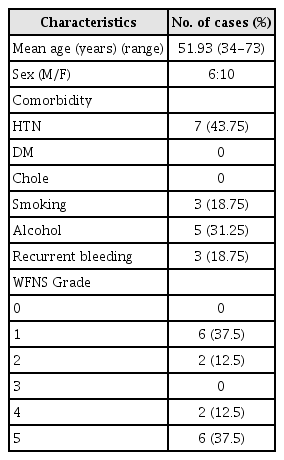
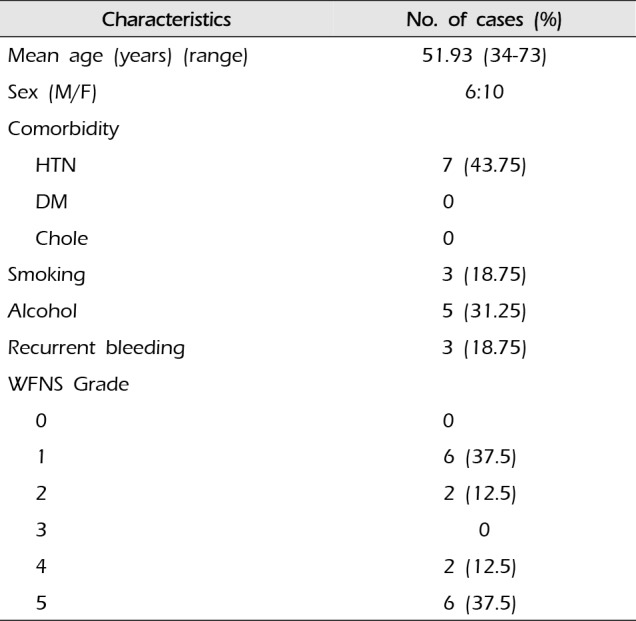
A total of 16 patients were included. There were 6 men and 10 women in this study, with age ranging from 34 to 73 years (mean age, 51.93 years). All patients presented with spontaneous SAH. The WFNS grades were as follows: 6 patients with grade I SAH, 2 patients with grade II SAH, 2 patients with grade IV SAH, and 6 patients (37.5%) with grade V SAH. The characteristics of the patients are shown in Table 1. The aneurysmal size ranged from 3 mm to 18 mm (mean size, 11.25 mm). There were 15 fusiform aneurysms and one saccular aneurysm. The right VA was affected by 12 aneurysms and the left VA was affected by 4 aneurysms. The aneurysms were located proximal to the PICA in 1 patient and distal to the PICA in 8 patients, and they involved the PICA in 6 patients and the anterior spinal artery in one patient.
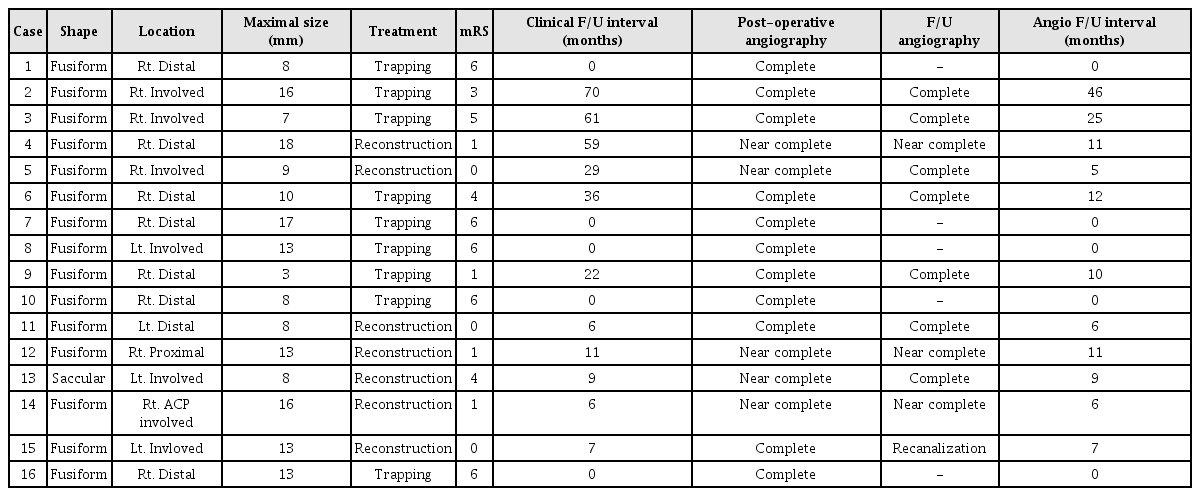
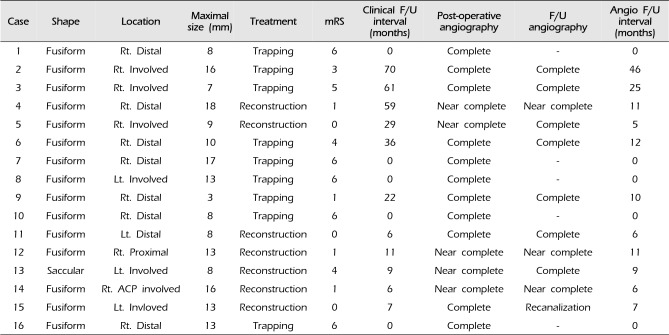
Endovascular treatment was technically successful in all aneurysms of these 16 patients. There were no procedural complications including rupture of an aneurysm and a thromboembolic or hemorrhagic event in any of the patients. In all patients, endovascular treatment was performed on the day of admission. Three patients (18.75%) suffered recurrent bleeding during the preoperative period. Among these 3 patients with recurrent bleeding, 2 patients died. Three other patients died due to severe brain stem injury. A total of 5 patients (31.25%) expired. For these 16 aneurysms that were treated endovascularly, an internal trapping procedure or occlusion of the parent artery was performed in 9 patients, compared with vascular reconstruction using stent-assisted coiling in 7 patients. Analysis of the immediate post-operative angiographic outcome indicated complete occlusion (Fig. 1) in 11 patients and near complete occlusion such as contrast stagnation in the neck of the aneurysms in 5 patients. Six aneurysms involved the PICA and the modalities of treatment used were aneurysm trapping in 3 patients and vascular reconstruction in 3 patients.

Angiogram of a 48-year-old female who presented with ruptured right intracranial VA dissecting aneurysm. Anteroposterior (A) and lateral (B) angiograms of the right VA reveal a string sign and fusiform aneurysm formation distal to the PICA. Anteroposterior (C) and lateral (D) angiograms of the VA obtained after telescopic stents and coil embolization demonstrating complete occlusion of the dissecting aneurysm. VA = vertebral artery; PICA = posterior inferior cerebellar artery.
The mean duration of follow-up was 28.73 months (range, 6–70 months). Clinical outcomes were favorable in 7 of 11 patients. The unfavorable outcome rate (mRS > 2), except for the patients who expired, was 36%. Of these patients, 2 developed symptomatic medullary infarction; one patient developed PCA territory infarction on follow-up CT and one patient suffered hemiparesis. The overall mean mRS for survivors was approximately 1.8. Angiographic follow-up was available for all survivors at a mean period of 13 months (range, 6–46 months). Complete occlusion of the aneurysm was achieved in 9 patients. Seven patients showed near complete occlusion on angiography. Only one patient showed recanalization of the VA dissecting aneurysm on follow-up angiography after 7 months (Fig. 2). Aneurysmal profile, endovascular treatment, and follow-up are summarized in Table 2.
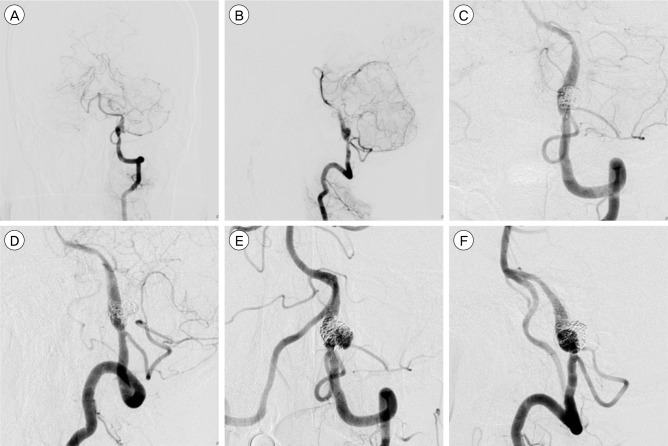
Images of a 55-year-old man who presented with ruptured left intracranial VA dissecting aneurysm. Anteroposterior (A) and lateral (B) angiograms of the right VA reveal a string sign and fusiform aneurysm formation distal to the PICA. Working view (C), (D) angiogram of the VA obtained after stent-assisted coil embolization demonstrating complete occlusion of the dissecting aneurysm. Anteroposterior (E) and lateral (F) angiograms of the right VA show recanalization at the previous treatment site after 7 months. VA = vertebral artery; PICA = posterior inferior cerebellar artery.
DISCUSSION
In 1977, Yonas first described the pathologic and radiographic appearance of a dissecting aneurysm.23) VA dissection is an intimal tear in the wall of the VA leading to intrusion of blood within the layers of an arterial wall. When blood enters the split layer, the result is a ‘pear and string sign’ on angiography. Because the intradural VA has a thin media and adventitia with fewer elastic fibers, dissection of the intradural VA is more likely to result in the formation of a pseudoaneurysm compared with dissection of the extradural VA. Various pathologic findings with respect to VA dissection have been reported, many of which indicate idiopathic medial necrosis.15) However, Mizutani et al. indicated that injury to the intima was the predominant factor.10) They found that lesions could be categorized based on whether the lesions had an entrance only or they had an entrance and an exit through the intima communicating with the pseudoaneurysm. An animal model has been developed based on chemical injury to the intima, leading to dissecting aneurysms in the aorta of rats.2)13)
Intracranial VA dissecting aneurysms are rare lesions. However, advances in imaging modalities and brain imaging screening have increased the rate of detection of intracranial VA dissecting aneurysms. In relative terms, unruptured intracranial VA dissecting aneurysms usually have a benign course.3)25) Nevertheless, the mortality rate in treated patients was reported to be 20%, whereas in untreated patients, it was 50%.16) The prognosis of a ruptured intracranial VA dissecting aneurysm is very poor. The most common cause is high incidence of recurrent bleeding at an early stage. Approximately 30–70% of patients with ruptured intracranial VA dissecting aneurysms will have recurrent bleeding.1)4)11)21)22) The other cause of poor prognosis is the clinical grade on admission. Approximately 50% of ruptured intracranial VA dissecting aneurysms had a poor WFNS grade (4 and 5).19) However, the clinical course of poor WFNS grade SAH caused by ruptured intracranial VA dissecting aneurysms might be more promising than SAH caused by ruptured saccular aneurysms.19)
In most of the cases, VA dissecting aneurysms have a broad neck and fragile walls. Therefore, surgical clipping or endovascular coil embolization is difficult. A variety of treatment strategies have been applied in patients with VA dissecting aneurysms including surgical reconstruction, surgical ligation, wrapping, surgical bypass, and endovascular treatment. Advances in endovascular treatment have led to a change in the treatment options for VA dissecting aneurysms; however, optimal treatment strategies have not yet been established. Various endovascular treatments have been used to treat VA dissecting aneurysms. Approaches to endovascular treatment of VA dissecting aneurysms can be divided into reconstructive and deconstructive techniques. Reconstructive endovascular techniques restore the normal vascular anatomy and preserve the parent artery; these techniques use single or double stent placement with or without coil embolization. Lanzino et al.9) and Park et al.14) reported about the effectiveness of treatment with a single stent. Stent placement also has other advantages such as altering the hemodynamics and attaching the intimal flap to the vessel wall. When the VA dissecting segment involves the PICA or the anterior cerebral artery or when the collateral flow in the involved artery is inadequate, such skills are more available. However, endovascular treatment using stents usually does not prevent recurrent bleeding.7)24) On the other hand, deconstructive endovascular techniques are available for occlusion of the parent artery or for trapping the aneurysms. But, other literatures have shown that proximal occlusion did not completely eliminate the risk of recurrent bleeding.1)5) The main concern with endovascular occlusion of the parent artery is the possibility of thrombus propagation into the PICA even if not it is included in the dissected segment and the perforators originating from the vertebral artery. The most complete treatment of a VA dissecting aneurysm is occlusion of the parent artery. There are several limitations to this technique. For patients with hypoplastic contralateral VA, PICA-involved VA dissecting aneurysms, bilateral lesions, or lesion of the extended basilar artery, this treatment results in not only cerebellar infarctions but also medullary infarctions. Therefore, reconstructive endovascular techniques are needed in these cases.
In this study, three patients with ruptured intracranial VA dissecting aneurysms suffered recurrent bleeding in the hyperacute stage. The rate of recurrent bleeding was 18.75% during the preoperative period. Five patients died and the mortality rate was 31.25%, and the unfavorable outcome rate for survivors was 36%. Our series confirmed that ruptured intracranial VA dissecting aneurysms were associated with a higher recurrent bleeding rate, severe morbidity and mortality, as reported in other literatures. Among the 6 patients with ruptured intracranial VA dissecting aneurysms involving the PICA, 3 patients were treated with deconstructive techniques and 3 patients were treated with reconstructive techniques. All 3 patients treated with deconstructive techniques suffered severe disability. Two out of the 3 patients treated with reconstructive techniques showed good recovery. It is noteworthy that the reconstructive technique is useful in the treatment of ruptured intracranial VA dissecting aneurysms involving the PICA. Sixteen patients with ruptured intracranial VA dissecting aneurysms were successfully treated by endovascular treatment. Of these, 9 patients were treated with deconstructive techniques and 7 patients were treated with reconstructive techniques. There were no procedure-related complications. During the follow-up, recurrent bleeding was not observed. Only one patient showed recanalization of the VA dissecting aneurysm on follow-up angiography after 7 months. Thus, endovascular treatment may be a useful option for ruptured intracranial vertebral artery dissecting aneurysms.
CONCLUSION
Ruptured intracranial vertebral artery dissecting aneurysms are associated with poor functional outcome and high mortality. Because of a high recurrent bleeding rate, more immediate treatments are needed. In addition, endovascular treatment may be a useful option for ruptured intracranial vertebral artery dissecting aneurysms.
Notes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.