 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 20(3); 2018 > Article |
|
Abstract
Objective
Ischemic postconditioning (IPostC), consisted of transient brain ischemia/reperfusion cycles, is considered to have neuroprotective effect. However, there is no best single protocol of IPostC, because varied factors like species tested and characteristics of the tissue may affect the efficacy of IPostC. Thus, we investgated whether different protocols of IPostC affect neuroprotective effects in experimental animal models.
Materials and Methods
Through occlusion of middle cerebral artery (MCA) with intraluminal suture, stroke was induced in a transient focal ischemia model in mice. We conducted IPostC via brief and repeated MCA occlusion, 2 minutes after reperfusion, followed by different ischemia and reperfusion protocols. After procedure, functional neurological score and histological examination were evaluated.
A cerebral vascular accident also known as stroke is the second leading cause of death worldwide and well known for the major cause of acquired disability.1) Despite of the high mortality and morbidity caused by stroke, efficient clinical treatments remain limited at present. The only approved treatment for acute ischemic stroke is the injection of alteplase via intra-venous within 4.5 hours,12) yet this acute injury is followed by inevitable ischemia/reperfusion injury. Thus, it is still an urgent task for many physicians to develop novel treatments to improve the outcomes in patients with acute ischemic stroke.
The concept of ischemic postconditioning (IPostC) refers to the performance of short cycles of ischemia followed by short cycles of reperfusion immediately after ischemia.27)
Most, but not all experimental studies investigating IPostC have demonstrated protective effects of IPostC. However, there is no consensus in the literature between different organs, because cell metabolism might influence the optimal number of cycles and their ischemic duration. It is well known that short cycles of IPostC are more neuroprotective than long cycles.2)4)17)21)27) However, other differences in the IPostC protocol may also be important in many clinical settings, especially regarding the number or duration of IPostC cycles.11)13)19) In clinical experiments, the duration of occlusions, the effect of the number of cycles, and the effector organ mass on the cardioprotective efficacy of remote IPostC remains unknown largely and too much conditioning, termed ŌĆśhyperconditioningŌĆÖ, with an excessive number of conditioning cycles has been shown to exert detrimental effects.23)
Therefore, we investigated the effect of the number of cycles and the ischemic duration within each IPostC and the infarction mass size on the efficacy of neuroprotection. Furthermore, the long-term effect of IPostC on neuroprotection was evaluated through the functional neurological score.
To evaluate effect of the number of cycles and the duration of each cycle on the effectiveness of IPostC, this study was designed.
This study was classified into 3 sub-studies as described following: the first subgroup (subgroup I) aimed to investigate whether the number of cycles applied is more important for neuroprotection than the duration of the cycles. Different protocols of 1, 3, 5 or 10 cycles of occlusion/reperfusion were performed on different animals. The second subgroup (subgroup II) aimed to evaluate whether the reperfusion cycle or the ischemic cycle is more important for neuroprotection. Two different stimuli with cycles of 15 seconds ischemia/30 seconds reperfusion or 30 seconds ischemia/15 seconds reperfusion were performed on different animals. The last subgroup (subgroup III) aimed to confirm the long-term effects of IPostC on infarction size and functional neurological score. Mice were sacrificed 14 days after surgery.
The Chonnam National University Hospital ethical committee approved all protocols used in this experimental study. Experiments were performed on male C57BL/6 mice between 8-10 weeks of age, weighing 22-25 g. Animals were purchased from Samtako Bio Korea (Osan, Korea), given a standard water and food ad libitum. In a temperature-controlled environment, 12-hour light/12-hour dark cycle was maintained. To reduce the number of mice sacrificed and to minimize the pain that the mice underwent, endeavors were made. Anesthesia was induced with 5% isoflurane and maintained with 1% to 2% isoflurane throughout the duration of surgery. The core temperature of body was checked by using a rectal probe and kept constant at 37 ┬▒ 0.5Ōäā with a surface warming pad for the entire surgery. Focal ischemia was produced as previously described with slight modifications.24) Using the operating microscope, exposure of left common carotid artery (CCA) and external carotid artery (ECA) was perfomed via a midline incision on ventral neck. And then the proximal CCA and ECA were ligated. At right below the carotid bifurcation, a 6-0 silicon-coated nylon suture with a 0.23 mm tip diameter (Doccol, Redlands, CA, USA) was inserted via the incision on the CCA and moved upward approximately 8 ┬▒ 0.5 mm. when a resistance was slightly felt, the advance stopped. To fix the suture in position, using 5-0 black silk suture, the inserted 6-0 nylon suture was secured at the proximal CCA bifurcation. In the ischemic control group, reperfusion was performed by permanent withdrawal of the suture after 60 minutes of occlusion.
IPostC was performed by short, repetitive occlusions of middle cerebral artery (MCA) through insertion and withdrawal of the suture into the internal carotid artery (ICA). To perform IPostC, the suture was moved back approximately 2 to 3 mm to allow temporary reperfusion and then re-inserted. Two minutes after reperfusion, IPostC with 15 seconds occlusion and 30 seconds reperfusion was started.
The mice were sacrificed three days after stroke, the volume of infarction was evaluated using 2,3,5- triphenyltetrazolium chloride (TTC) staining, and determined as a percentage of the contralateral hemisphere using the following formula: (contralateral hemispheric volume - ipsilateral non-infarcted hemispheric volume / contralateral hemispheric volume) ├Ś 100%.
FNS were assessed using a 28-point neuroscore test9) that was carried out by an experimenter who was blinded to the experimental groups at 1, 2, 3, 5, 7, 10, and 14 days. The mice were evaluated in seven tests. These tests include body symmetry, gait, climbing at angle of 45 degrees, circling behavior, front limb symmetry, compulsory circling and whisker response. The 5-point scale (0 to 4) was used in each test. The total score was calculated by sum of individual test score.15)
TTC staining is not suitable for measuring the infarction size in the chronic stages of stroke because the borderline between the infarcted and normal brain is not clear due to live mitochondria. The silver staining is a modified neurofibril staining method.6)22) For silver staining, a silver impregnation solution shaken vigorously for 1 minute was prepared and then slides were submerged into the solution for 2 minutes. The slides were then washed in distilled water six times for 1 minute before they were moved into developer solution which was shaken vigorously for 3 minutes. After washing the slides three times for 1min in distilled water, they were dried in air. The preparation procedures and composition of the developer solution and impregnation were as follows.
In 5 mL of a 10% silver nitrate solution, 10 milliliters of a saturated lithium carbonate solution was added. To dissolve the precipitate, adding of a 25% ammonia solution (500 mL) in drops and continuous stirring were performed until the solution became clear. After adding 75 mL of distilled water, the solution was placed under darkness until use. The most critical factor in the total staining procedure is the addition of ammonia. While fine remnants of the precipitate do not disrupt the reaction, an excess of ammonia lead to the staining failure.
In 70 mL of distilled water, 20 milliliters of a 37% formaldehyde solution was mixed. Then addition of 15 mL acetone and 0.3 g hydroquinone was done, and the solution was agitated gently to dissolve the hydroquinone. Subsequently, we dissolved 1.1 g trisodium citrate dihydrate in the solution. The solution was then exposed to room air until it turned to cooper color (30 to 60 minutes). All solutions were made daily in carefully washed (65% nitric acid/distilled water) glassware.
All data were presented as mean ┬▒ scanning electron microscope. Data was analyzed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Statistical analysis of volumes of infarction was performed with analysis of variance (ANOVA) followed by Newman-Keuls post hoc or Dunnet's tests. sing one-way ANOVA Test (followed by Bonferroni post hoc test) FNS results were analyzed. p < 0.05 was considered significant statistically.
IPostC was induced by brief and repeated occlusion/ reperfusion 2 minutes following suture withdrawal after 60 minutes of ischemia. We have previously identified that IPostC with 15 seconds occlusion/30 seconds reperfusion that was initiated 2 minutes after 60 minutes of MCA occlusion produced stronger neuroprotection than was observed in other protocols.15) Nevertheless, the protective effects of different numbers of cycles has not been investigated in the IPostC model. Therefore, in this study, we designed different protocols to compare the protective effects of IPostC initiated 2 minutes after 60 minutes of MCA occlusion, when 1, 3, 5, or 10 cycles of 15 seconds occlusion and 30 seconds reperfusion were performed (Fig. 1). The effect of protocols using different numbers of cycles of IPostC on infarct size are shown in Fig. 2. The protocol that consisted of 3 cycles of IPostC significantly reduced the infarction size to 27.94 ┬▒ 2.92% (control level = 48.21 ┬▒ 3.44%, p = 0.003 vs. control). Whereas, the infarction size was not significantly reduced following 1 cycle of IPostC (39.32 ┬▒ 4.88%, p = 0.14), 5 cycles of IPostC (35.86 ┬▒ 4.86%, p = 0.06), or 10 cycles of IPostC (46.58 ┬▒ 5.07%, p = 0.79). In summary, A protocol that consists of 3 cycles of IPostC demonstrated the strongest neuroprotection. However, protocols that consisted of 1, 5, or 10 cycles of IPostC did not significantly reduce the size of the infarction. The result have suggested that the more number of cycles of IPostC over a defined cycles (3 cycles) and only 1 cycle of IPostC decline the neuroprotective effects and by increasing number of cycles, it can lost the effect eventually.
Group I was achieved through the permanent withdrawal of the suture after MCA occlusion for 60 minutes. In group II, IPostC was performed 2 minutes after MCA occlusion for 60 minutes with 3 cycles of 15 seconds occlusion and 30 seconds reperfusion. In grmaoup III IPostC was initiated 2 mintues after MCA occlusion for 60 minutes with 3 cycles of 30 seconds occlusion and 15 seconds reperfusion (a reversal of the protocol used in group II; Fig 3A). Compared to control (48.21 ┬▒ 3.44%), both group II and III show a significant reduction in infarction size (28.45 ┬▒ 3.54%, p = 0.005 and 34.15 ┬▒ 4.15% p = 0.03, respectively Fig. 3B, C).
The infarction size and functional neurological score were measured. Mice were sacrificed 14 days after surgery. In control group, the infarction size at day 3 was significantly larger than that of the IPostC group which consist of 3 cycle of protocol with 15 seconds occlusion/30 seconds reperfusion that was initiated 2 minutes after MCA occlusion for 60 minutes, as estimated by TTC staining. In the TTC staining, the viable tissue turn to red because of the activity of mitochondrial dehydrogenases (Fig. 2A, 3B).
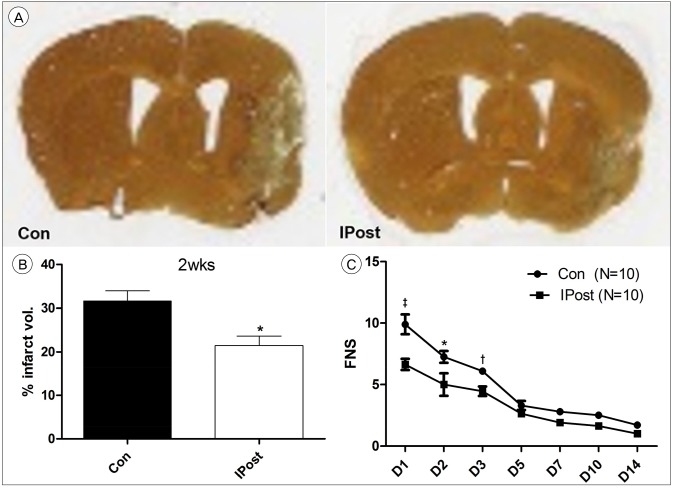
The difference in infarct size was still observed 14 days after the stroke, as estimated by silver staining (used to identify degeneration of neurons and axons; Fig. 4A). The IPostC group had smaller infarction size, compared to control (32.82 ┬▒ 4.54% vs. 24.34 ┬▒ 3.24%, p = 0.02; Fig. 4B). The FNS in the IPostC group were much better than control group, especially at the acute stage (days 1, 2, and 3). The difference in scores between the two group was reduced after day 5 (Fig. 4C).
IPostC is described in many clinical or experimental researches as a simple and safe method to enhance protective effect against ischemia/reperfusion injuries to various organs, such as the brain, spinal cord, heart, liver, kidney, lung, intestine and skeletal muscle.18)28) Despite IPostC has the effectiveness in various organs in humans and experimental models, there is currently no standardized stimulation method in preclinical and clinical studies. In application of IPostC, one of the main variables is the protocol; the number of IPostC stimuli, and the duration of each episode. Because different protocols of IPostC may lead to different effects in a certain organ, we cannot expect the same protocol to evoke similar effects in different organs. Namely, the best protocol that can be applied for all species and various organs has not yet been established.
The present study demonstrates that mainly the number of cycles of IPostC, but also the duration of ischemic cycles determine the efficacy of IPostC. However, there was no statistically significant neuroprotection following IPostC consisting of fewer than or more than 3 cycles in this study. IPostC refers to a series of brief cerebral blood vessel occlusions, performed during reperfusion following an ischemic event, that trigger endogenous neuroprotective mechanisms in cerebral ischemia.26) The concepts of ischemic preconditioning (IPreC) and IPostC have expanded to represent a broad range of sub-lethal insults, from ischemia, neurotoxic agents and pharmacological agents, to physical exercise.7)8)26) The main goal of studying IPostC in stroke is for clinical application to patients.25) Despite the fact that none of the patterns of IPostC have yet been successfully established to apply for the stroke patients after decades of study, the concept of IPostC is still relatively new and there is potential for clinical application. Therefore, finding novel strategies to reduce the infarction size following stroke and to improve patient outcome is clinically very important. Keeping these concerns in mind, we intended to introduce the concept of hormesis in IPostC against stroke.
Such a phenomenon was first described by Hugo Schulz, a pharmacologist in Germant, in the 1880s after observing that small dose of poisons could stimulate the growth of yeast. The concept of ŌĆ£hormesisŌĆØ was established for the first time in a scientific literature by Calabrese3) The dose-response characteristics of IPostC originate from local ischemic preconditioning. Although an early study of local IPreC suggested that preconditioning was an all-or-nothing phenomenon with protection resulting from a single cycle,16) subsequent studies have pointed toward local ischemic preconditioning as a graded phenomenon with additional protection produced by supplementary cycles.10)20) However, several studies have reported an excessive number of IPreC cycles lead to a loss of protection.5)14)16) In our experiments, the results show that 3 cycles of IPostC provide the strongest protection, with 1, 5, and 10 cycles producing no significant reduction in infarction size. These results are consistent with the concept of a hermetic response. Again, the strongest protection is achieved through 3 cycles of IPostC and a decrease or an increase in the number of cycles performed may lead to a loss of protection.23) In fact, if the number of cycles of IPostC increase it will lead to cumulative damage and detract from the overall benefits of IPostC. However, the present study does not explain why this phenomena happens. One possibility is that the multiple cycles of ischemia lead to loss of protection directly. The other possibility is that the protection of IPostC occurs as ever, however the accumulated injury by the IPostC protocol counteracts the neuroprotection. Our findings are in line with the theory that multiple ischemic preconditioning episode are not protective.14) However, we should be careful to interpret this result because there is a possibility that the more cycles of IPostC performed, the time of anesthesia would be longer. Thus this may influence the infarction size.
We strongly encourage the development of more well-designed studies to deter-mine certainly whether IPreC and IPostC follow concept of hormesis. This may promote the discovery of alternative tools for inducing preconditioning and postconditioning against infarction including ischemic stroke. We think these results should be considered when candidates are chosen for stroke treatment. We evaluated reduction of infarction size as the primary endpoint for neuroprotection. Accordingly, reduction of infarction size is usually paralleled by an improvement in neurological functional status (Fig. 4C).
The duration of acute brain ischemia is one of the main and primary determinants of the development of infarction size. However, there is no literature, among the basic and clinical studies after stroke, regarding whether reperfusion cycles or ischemic cycles are more important factors in IPostC-induced neuroprotection. We have previously compared the protective effects of IPostC of various protocols in this mouse model. The IPostC protocols tested include onset times from 0 second to 3 hours after reperfusion, and different periods of occlusion or reperfusion in each cycle. We identified that an IPostC protocol with 15 seconds occlusion/30 seconds reperfusion that was initiated 2 minutes after 60 minutes of MCA occlusion produced the strongest protection compared with other protocols.15) Thus, in this study, we investigated the infarction size following the use of a reverse algorithm with 30 seconds occlusion/15 seconds reperfusion. The neuroprotective effect of IPostC was still significant but slightly decreased.
As previously discussed, the ischemic patterns of IPostC are very important because different tissues can withstand a certain period of ischemia. However, IPostC protocols should be designed based on the type of tissues or organ in the species used. The factors to be considers include the tissues' requirements for nutrients, the vascular blood flow, and the metabolic status of the tissue. One protocol may have very good protection in one tissue, but may have a moderate to low protective effect in another tissue, and may even have a negative effect elsewhere. Thus, we cannot easily optimize a single protocol for use in any tissue, because of the diversity in the physiological requirements between tissues. The pursuit of further understanding of the mechanisms through with the IPostC protocol confers neuroprotection against cerebral ischemia may potentially move its clinical translation forward and shed new light on the discovery of novel therapeutic targets.
We only studied whether different protocols of IPostC affect neuroprotective effects in experimental animal models. Therefore, the weakness of this observational study is that the novelty of study methods should be improved, and mechanistic study should be added in the future.
The effects of IPostC are definitely affected by differences in the protocol used, including the number of cycles, the duration of individual ischemia/reperfusion episode and the entire duration of the IPostC stimuli. However, the different features of distinct tissues should be considered when designing an optimal working IPostC protocol for the protection of a certain organ against ischemia/reperfusion insults.
ACKNOWLEDGEMENTS
This work was supported by a grant (2013-CURIMS-DR006) from the Research Institute of Medical Sciences, Chonnam National University.
References
1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017 3;135(10):e146-e603;



2. Bretz B, Blaze C, Parry N, Kudej RK. Ischemic postconditioning does not attenuate ischemia-reperfusion injury of rabbit small intestine. Vet Surg. 2010 2;39(2):216-223;


3. Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008 7;27(7):1451-1474;


4. Chu W, Li S, Wang S, Yan A, Nie L. Ischemic postconditioning provides protection against ischemia-reperfusion injury in intestines of rats. Int J Clin Exp Pathol. 2015 6;8(6):6474-6481;


5. Cohen MV, Yang XM, Downey JM. Conscious rabbits become tolerant to multiple episodes of ischemic preconditioning. Circ Res. 1994 5;74(5):998-1004;


6. D'Amelio FE. The Golgi-Hortega-Lavilla technique, with a useful additional step for application to brain tissue after prolonged fixation. Stain Technol. 1983 3;58(2):79-84;


7. Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009 4;8(4):398-412;



8. Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003 5;26(5):248-254;


9. Doyle KP, Yang T, Lessov NS, Ciesielski TM, Stevens SL, Simon RP, et al. Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke. J Cereb Blood Flow Metab. 2008 6;28(6):1235-1248;



10. Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995 9;77(3):611-621;


11. Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000 4;41(4):493-496;


12. Hacke W, Kaste M, Bluhmki E, Brozman M, D├Īvalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 9;359(13):1317-1329;


13. Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, et al. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur Heart J. 2014 1;35(3):176-183;



14. Iliodromitis EK, Kremastinos DT, Katritsis DG, Papadopoulos CC, Hearse DJ. Multiple cycles of preconditioning cause loss of protection in open-chest rabbits. J Mol Cell Cardiol. 1997 3;29(3):915-920;


15. Joo SP, Xie W, Xiong X, Xu B, Zhao H. Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience. 2013 7 23 243:149-157;



16. Li GC, Vasquez JA, Gallagher KP, Lucchesi BR. Myocardial protection with preconditioning. Circulation. 1990 8;82(2):609-619;


17. Lintz JA, Dalio MB, Joviliano EE, Piccinato CE. Ischemic pre and postconditioning in skeletal muscle injury produced by ischemia and reperfusion in rats. Acta Cir Bras. 2013 6;28(6):441-446;



18. Papadopoulos D, Siempis T, Theodorakou E, Tsoulfas G. Hepatic ischemia and reperfusion injury and trauma: current concepts. Arch Trauma Res. 2013 8;2(2):63-70;



19. Prasad A, G├Čssl M, Hoyt J, Lennon RJ, Polk L, Simari R, et al. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv. 2013 5;81(6):930-936;


20. Sandhu R, Diaz RJ, Mao GD, Wilson GJ. Ischemic preconditioning: differences in protection and susceptibility to blockade with single-cycle versus multicycle transient ischemia. Circulation. 1997 8;96(3):984-995;


21. Santos CH, Gomes OM, Pontes JC, Miiji LN, Bispo MA. The ischemic preconditioning and postconditioning effect on the intestinal mucosa of rats undergoing mesenteric ischemia/reperfusion procedure. Acta Cir Bras. 2008 Jan-Feb;23(1):22-28;



22. Vogel J, Mobius C, Kuschinsky W. Early delineation of ischemic tissue in rat brain cryosections by high-contrast staining. Stroke. 1999 5;30(5):1134-1141;


23. Whittaker P, Przyklenk K. From ischemic conditioning to ŌĆśhyperconditioningŌĆÖ: clinical phenomenon and basic science opportunity. Dose Response. 2014 12;12(4):650-663;



24. Xiong X, Gu L, Zhang H, Xu B, Zhu S, Zhao H. The protective effects of T cell deficiency against brain injury are ischemic model-dependent in rats. Neurochem Int. 2013 2;62(3):265-270;


25. Zhao H. Hurdles to clear before clinical translation of ischemic postconditioning against stroke. Transl Stroke Res. 2013 2;4(1):63-70;




26. Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009 5;29(5):873-885;



Fig.┬Ā1
Experimental protocols. Focal ischemia was induced by of temporary occlusion of middle cerebral artery for 60 minutes. This study compared the protective effects of IPostC with 1, 3, 5, and 10 cyclese of 15 seconds occlusion/30 seconds reperfusion initiated 2 minutes post-reperfusion. Group C is the ischemic control, without IPostC. IPostC was initiated 2 minutes after reperfusion, and consisted of a differing number of cycles of 15 seconds occlusion/30 seconds reperfusion. N = 10 mice/group. IPostC = ischemic postconditioning.

Fig.┬Ā2
TTC staining and infarction volume. (A) TTC staining from representative examples of infarctions are shown in the top panel. (B) The bar graph shows the average infarct volume in each group. IPostC with 3 cycles provided the strongest protection, while IPostC with 1, 5, or 10 cycles did not sufficiently reduce the infarction size. Data are showed by the mean ┬▒ scanning electron microscope. N = 10 mice/group. TTC = triphenyltetrazolium chloride; IPostC = ischemic postconditioning. *p < 0.05; ŌĆĀp < 0.01 compared to control group.

Fig.┬Ā3
Experimental protocols, TTC staining and infarction volume. (A) Focal ischemia was induced by 60 minutes of transient middle cerebral artery occlusion. Group I is the ischemic control without IPostC (n = 10). Group II was initiated 2 minutes after reperfusion, 3 cycles of 15 seconds occlusion/30 seconds reperfusion were performed (n = 10). Group III was initiated 2 minutes after reperfusion, 3 cycles of 30 seconds occlusion/15 seconds reperfusion (the reversal of the pattern used in group II) were performed (n = 10). (B) TTC staining from representative examples of infarctions are shown. (C) Graph shows the average volume of infarction in each group. IPostC, which did 3 cycles of 15 seconds occlusion/30 seconds reperfusion provided the strongest protection compared to 3 cycles of 30 seconds occlusion/15 seconds reperfusion. Data are presented as the mean ┬▒ scanning electron microscope. N = 10 mice/group. TTC = triphenyltetrazolium chloride; IPostC = ischemic postconditioning. *p < 0.05; ŌĆĀp < 0.01 compared to control group.
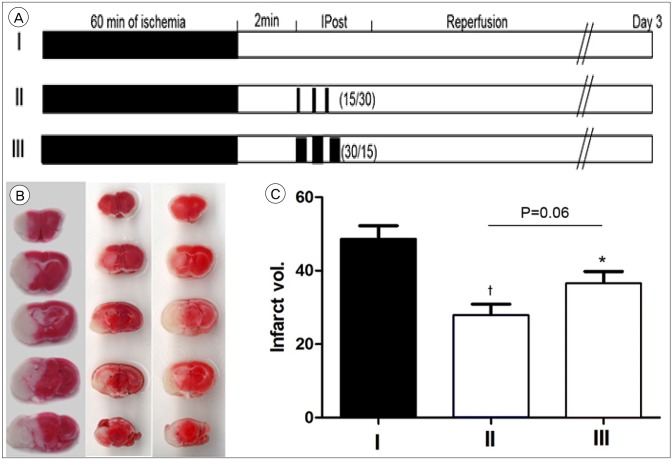
Fig.┬Ā4
The long-term effects of IPostC. (A) Silver staining from representative examples of infarctions are shown 14 days after stroke. (B) Graph shows the average infarct volume between the two groups. (C) The histogram shows the average FNS. severe scores mean greater impairment. Both 1 day and 3 day after reperfusion, FNS of all IPostC groups was improved significantly, compared with the FNS of control group FNS. Con = control; IPost = ischemic postconditioning; FNS = focal neurological scores; IPostC = ischemic postconditioning. *p < 0.05. ŌĆĀp < 0.01. ŌĆĪp < 0.001.
- TOOLS
-
METRICS

-
- 4 Crossref
- 0 Scopus
- 2,854 View
- 24 Download
- Related articles




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



