|
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 20(4); 2018 > Article |
|
Abstract
Treatment of paraclinoid aneurysms weather by surgery, or endovascular embolization has a risk of visual loss due to optic neuropathy, or diplopia due to cranial nerve palsies. Visual complications occur immediately after the clipping, whereas they can occur variable time after endovascular coiling. Recently, endovascular coiling for paraclinoid aneurysm is regarded as a safe and feasible treatment. But it still has risks of acute thromboembolic complication, or cranial nerve palsies. A 45-year-old woman was referred from local hospital to our hospital due to ruptured large ICA dorsal wall aneurysm. A total of 12 coils (195 cm) were used for obliteration of aneurysm. Postoperative diffusion weighted image showed no abnormal signal intensity lesion and magnetic resonance angiography demonstrated no sign of vasospasm, or vessel narrowing. But, she complained visual problem 23 days after coil embolization. Ophthalmologist confirmed the left optic disc atrophy on fundoscopy. Although steroid was started, but monocular blindness did not recover completely. The endovascular embolization of paraclinoid aneurysm, especially projecting superiorly with large irregular shape, has the risk of progressive visual loss because of the proximity to optic nerve.
Paraclinoid aneurysms sometimes have the neuroophthalmic signs and symptoms because of the proximity to the optic apparatus. These include visual loss due to optic neuropathy or diplopia due to ocular motor nerve palsy.10)
They have the high risk of visual complications after surgical clipping. Visual problems are made immediately after the clipping. Recently, endovascular coiling for paraclinoid aneurysm is regarded as a safe and feasible treatment. But it still has a risk of acute thromboembolic complication. In general, thromboembolism occurred on the site of stenting, parent artery, or perforating artery immediately in acute phase.6) On the other hands, delayed and progressive visual loss after endovascular treatment has rarely been reported and it has another mechanisms. We introduce one case of delayed visual loss after simple coiling of paraclinoid aneurysm for awakening the young endovascular neurosurgeon.
A 45-year-old woman was referred from local hospital to Soonchunhyang University Cheoan Hospital via emergency department due to severe headache. It was abruptly started during the work and was not relieved with medication. She has no medical history. On physical and neurologic examination, she had severe neck stiffness and no neurologic deficit (Hunt and Hess grade 2).
Initial brain computed tomography showed spontaneous subarachnoid hemorrhage (Fig. 1). Digital subtraction angiogram showed left internal carotid artery (ICA) dorsal wall aneurysm (neck, 4.7 mm; width, 11.7 ├Ś 9.4 m; height, 12.8 mm) directing superiorly and superior hypophyseal aneurysm (neck, 3.7 mm; width, 4.5 ├Ś 3.6 mm; height, 2.7 mm) directing infero-medially (Fig. 2). We regarded dorsal wall aneurysm as ruptured one because of the large size and irregular shape.
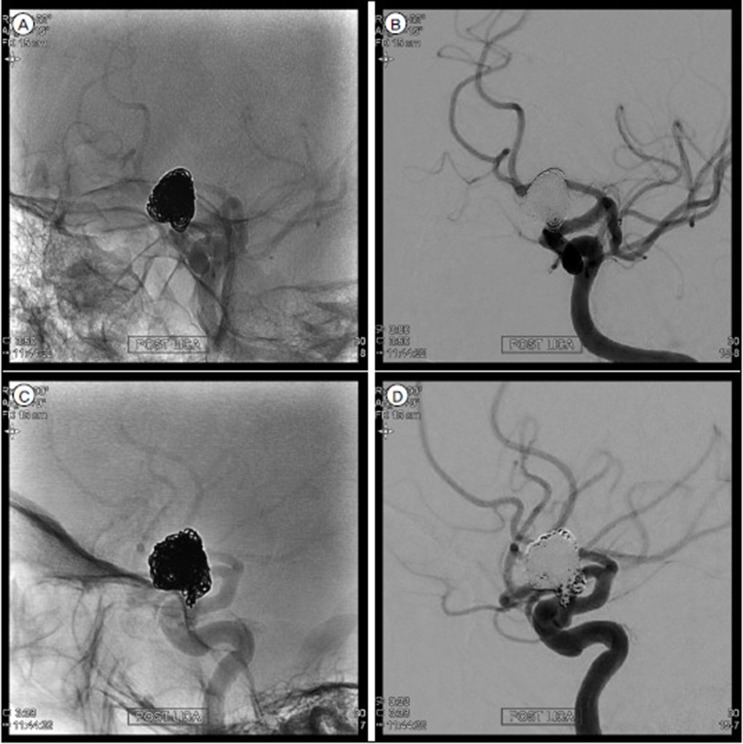
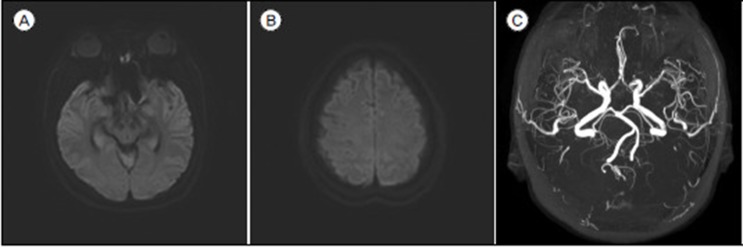
We decided to treat the ICA dorsal wall aneurysm with endovascular coil embolization. Headway microcatheter (Microvention, Tustin, CA, USA) was steam shaped like a pig-tail and it was positioned into the aneurysm dome portion without difficulty. An Axium (eV3; Medtronics, Irvine, CA, USA) 3D 10 mm/30 cm was employed as a framing coil and then 11 additional coils were continuously packed. A total of 12 coils (195 cm) were inserted. Final angiogram showed nearly complete obliteration of aneurysm with small neck remnant (Raymond II) (Fig. 3). Postoperative diffusion weighted image showed no abnormal high signal intensity lesion and magnetic resonance angiography demonstrated no sign of vasospasm, or vessel narrowing (Fig. 4). Gadolinium enhanced magnetic resonance image (MRI) was not taken because we could not expect the possibility of visual complication at that time. She was discharged home after 2 weeks without any complication.
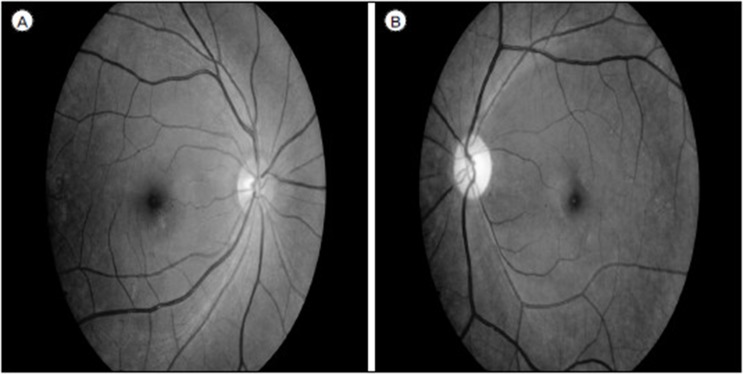
She visited outpatient clinic at 23 days after discharge. She complained visual problem at 23 days after coil embolization. Ophthalmologist confirmed the left optic disc atrophy on fundoscopy (Fig. 5). We started steroid (prednisolone 15 mg for 3 days and tapering for 9 days), but monocular blindness did not recover.
Paraclinoid aneurysms are vulnerable to have visual deterioration after both surgical and endovascular treatment because of their position near the anterior clinoid process and the dural ring.2)14)19) According to the type of treatment for paraclinoid aneurysm, the incidence and clinical course of visual symptom was varied. Beretta et al.2) reported that three of 38 patients (7.9%) after surgical clipping of paraclinoid aneurysms experienced new visual field deficits, and one of 12 patients (8.3%) after endovascular embolization had an acute onset of blindness requiring immediate surgical procedure to decompress the optic nerve. Vargas et al.22) reported 19 patients who had a visual loss before the endovascular treatment of giant aneurysm. Of these patients, 18 were improved or unchanged, and one (5.3%) experienced aggravation of visual loss after treatment. Hoh et al.8) reported no difference in comparing the visual loss with coiling versus clipping in the treatment of paraclinoid aneurysm. Delayed visual problem could be occurred after successful wrapping of fusiform aneurysm because of an excessive inflammatory reaction.3)18) Schmidt et al.16) reported eight patients of isolated progressive visual loss after coiling of paraclinoid aneurysm. Seven of eight patients (88%) had more than 10 mm diameters. Six of the eight patient had improvement in visual function with dexamethasone, but one patient had no change from nadir.
The reasons of visual loss after coil embolization of paraclinoid aneurysms can be categorized to the mechanism, timing, and acuity of the symptom onset. First, thromboembolism could provoke the sudden or progressive visual loss. Thromboembolic cause of visual loss is abrupt beginning and maximal at onset, related to direct retinal artery infarction or the ophthalmic artery occlusion. It could be possible due to occipital lobe infarction because of posterior communicating artery or posterior cerebral artery obstruction. These events are usually took place during the procedure or in the immediate post procedural time.6) These ischemic problems typically are not improved regardless of steroid therapy and may require emergent intervention to save visual ability.
Second, inflammation reaction could make the progressive visual loss. In our case, progressive and delayed monocular blindness occurs in 2 or 3 weeks after the procedure. Unlike the thrombotic events, which have an abrupt and maximal onset, these changes have a slower, more progressive onset. They are seems to have the evolving mass effect of aneurysm thrombosis and perianeurysmal inflammation/edema.13)21)23) The edema, the morphology of the aneurysm, its close relationship to the optic nerve, the mass effect, and visual condition before the embolization are the risk factors of the ultimate outcome of in these patients. But these visual problems could be stabilized or improved with use of corticosteroid in our case. On histopathology findings, inflammatory reactions mediated by numerous foreign body giant cells take place at the outer and inner surface of platinum coil when the aneurysm is packed. The chronic inflammation makes the aneurysm wall thickening and prevents aneurysm rupture in rabbit model.5) Bavinzski et al.1) studied 17 aneurysms in 15 patients who died after coiling. He found a polymorphonuclear leukocytic infiltration inner surface of aneurysm wall within 1 week. During the 9 and 14 days after embolization, fibroblasts invaded the wall. Granulation tissue and connective tissue proliferation and capillary ingrowth were present in aneurysms found on days 22 and 40. Surgically removed 54th month-old coil-packing aneurysm had thick fibrous tissue, collagen, small blood vessels larger than capillaries and coil loop invaded the aneurysm wall. These effects are favorable aspect of inflammation. Schmidt et al.16) see the radiologic evidence of inflammation, suggests that optic nerve damage and other cranial neuropathies are complication of chronic inflammation. Coil related inflammation reaction was also reported that the use of hydrocoil could related with occurrence of the aseptic meningitis and hydrocephalus than other type of coils.9)
Third, the degree of coil packing and configuration of aneurysm could affect the visual symptom. Enlargement of neck after coil embolization could make the neuroophthalmic complication.4) So, tightly packing with coils and no residual neck can reduce the water-hammer effect12) and make the coils not to change the configuration.7)11)15)20) Paraclinoid aneurysm with large size, wide neck or non-spheric configuration were another risk factors of delayed ophthalmic complication of coil embolization.16) Stracke et al.17) reported the case of patient dramatic improvement in vision when treated with systemic corticosteroids. They could see the enhancement and thickening of the optic chiasm just around the coil. But all this problems are disappeared after 1 week of dexamethasone.
Because visual deficit as postoperative complication is fatal, doctors should know possibility of this problem and inform the patient not to miss the golden time. In addition, when delayed and progressive visual loss occurs, we often miss the adequate evaluation and treatment as in our case. The evaluation of visual problem after coil embolization of dorsal wall aneurysm started with character of symptoms. If the thromboembolic problem was cause of blindness, patient present maximal-at-onset vision decline within hours after the embolization. Then we should perform the angiography to conform the patency of ophthalmic artery. If the visual loss was slow and progressive, gadolinium enhanced MRI could be used to evaluate the mass effect, edema, or inflammatory reaction and steroid therapy seemed to be useful treatment.
The endovascular embolization of paraclinoid aneurysm, especially with superior-directed large irregular shape has the risk of progressive vision loss because of the proximity to optic nerve. Both patient and physician should know about this fetal problem. Systemic steroid should be started before the problems come up. So we recommend prophylactic steroid therapy before and after procedure.
References
1. Bavinzski G, Talazoglu V, Killer M, Richling B, Gruber A, Gross CE, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999 8;91(2):284-293;


2. Beretta F, Andaluz N, Zuccarello M. Aneurysms of the ophthalmic (C6) segment of the internal carotid artery: treatment options and strategies based on a clinical series. J Neurosurg Sci. 2004 12;48(4):149-156;

3. Bhatti MT, Holder CA, Newman NJ, Hudgins PA. MR characteristics of muslin-induced optic neuropathy: report of two cases and review of the literature. AJNR Am J Neuroradiol. 2000 2;21(2):346-352;


4. Bhatti MT, Peters KR, Firment C, Mericle RA. Delayed exacerbation of third nerve palsy due to aneurysmal regrowth after endovascular coil embolization. J Neuroophthalmol. 2004 3;24(1):3-10;


5. Dai D, Ding YH, Kadirvel R, Danielson MA, Lewis DA, Cloft HJ, et al. A longitudinal immunohistochemical study of the healing of experimental aneurysms after embolization with platinum coils. AJNR Am J Neuroradiol. 2006 4;27(4):736-741;


6. Derdeyn CP, Cross DT 3rd, Moran CJ, Brown GW, Pilgram TK, Diringer MN, et al. Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 2002 5;96(5):837-843;


7. Fernandez Zubillaga A, Guglielmi G, Vi├▒uela F, Duckwiler GR. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994 5;15(5):815-820;


8. Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery. 2001 1;48(1):78-89; discussion 89-90.


9. Im SH, Han MH, Kwon BJ, Jung C, Kim JE, Han DH. Aseptic meningitis after embolization of cerebral aneurysms using hydrogel-coated coils: report of three cases. AJNR Am J Neuroradiol. 2007 3;28(3):511-512;


10. Kasner SE, Liu GT, Galetta SL. Neuro-ophthalmologic aspects of aneurysms. Neuroimaging Clin N Am. 1997 11;7(4):679-692;

11. Kawanabe Y, Sadato A, Taki W, Hashimoto N. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochir (Wien). 2001 143(5):451-455;


12. Kwan ES, Heilman CB, Shucart WA, Klucznik RP. Enlargement of basilar artery aneurysms following balloon occlusion--ŌĆ£water-hammer effectŌĆØ. Report of two cases. J Neurosurg. 1991 12;75(6):963-968;


13. Nakayama Y, Tanaka A, Ohshiro S, Yoshinaga S. Extensive edema in the thalamus caused by thrombosed basilar artery aneurysm. Neurol Med Chir (Tokyo). 1998 5;38(5):274-277;


14. Park HK, Horowitz M, Jungreis C, Kassam A, Koebbe C, Genevro J, et al. Endovascular treatment of paraclinoid aneurysms: experience with 73 patients. Neurosurgery. 2003 7;53(1):14-23;


15. Piotin M, Mandai S, Murphy KJ, Sugiu K, Gailloud P, Martin JB, et al. Dense packing of cerebral aneurysms: an in vitro study with detachable platinum coils. AJNR Am J Neuroradiol. 2000 4;21(4):757-760;


16. Schmidt GW, Oster SF, Golnik KC, Tumial├Īn LM, Biousse V, Turbin R, et al. Isolated progressive visual loss after coiling of paraclinoid aneurysms. AJNR Am J Neuroradiol. 2007 Nov-Dec;28(10):1882-1889;



17. Stracke CP, Krings T, M├Čller-Hartmann W, Mahdavi A, Klug N. Severe inflammatory reaction of the optic system after endovascular treatment of a supraophthalmic aneurysm with bioactive coils. AJNR Am J Neuroradiol. 2007 8;28(7):1401-1402;



18. Subramanian PS, Miller NR, Renard V, Tamargo RJ. Delayed progressive visual loss following wrapping of bilateral clinoidal aneurysms: recovery of vision and improvement in neuroimaging during corticosteroid treatment. Br J Ophthalmol. 2005 12;89(12):1666-1668;



19. Thornton J, Aletich VA, Debrun GM, Alazzaz A, Misra M, Charbel F, et al. Endovascular treatment of paraclinoid aneurysms. Surg Neurol. 2000 10;54(4):288-299;


20. Turjman F, Massoud TF, Sayre J, Vi├▒uela F. Predictors of aneurysmal occlusion in the period immediately after endovascular treatment with detachable coils: a multivariate analysis. AJNR Am J Neuroradiol. 1998 10;19(9):1645-1651;


21. Ushikoshi S, Kikuchi Y, Houkin K, Miyasaka K, Abe H. Aggravation of brainstem symptoms caused by a large superior cerebellar artery aneurysm after embolization by Guglielmi detachable coils--case report. Neurol Med Chir (Tokyo). 1999 7;39(7):524-529;


Fig.┬Ā1
(A) Initial brain computed tomography. (B) Brain angio-computed tomography showing the large aneurysm on right paraclinoid internal carotid artery.
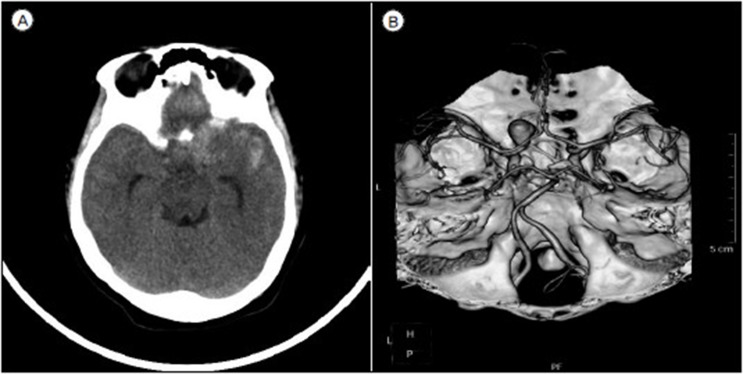
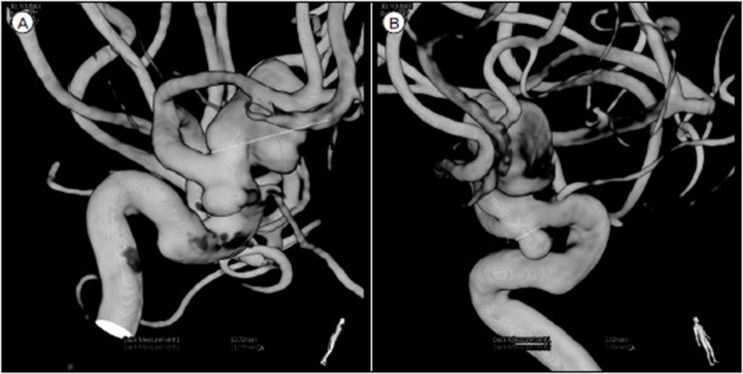
Fig.┬Ā2
(A) Maximal size with 12.8 mm with superior direction, (B) maximal size with 4.5 mm with inferior direction.
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,511 View
- 30 Download
- Related articles
-
Hemodynamic Infarction Associated with Coil Embolization of Intracranial Aneurysm.2003 March;5(1)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print