Optimal Surgical Timing of Aspiration for Spontaneous Supratentorial Intracerebral Hemorrhage
Article information
Abstract
Objective
Minimally invasive techniques such as stereotactic aspiration have been regarded as promising alternative methods to replace craniotomy in the treatment of intracerebral hemorrhage (ICH). The aim of this study was to identify the optimal timing of stereotactic aspiration and analyze the factors affecting the clinical outcome.
Materials and Methods
This retrospective study included 81 patients who underwent stereotactic aspiration for spontaneous supratentorial ICH at single institution. Volume of hematoma was calculated based on computed tomography scan at admission, just before aspiration, immediately after aspiration, and after continuous drainage. The neurologic outcome was compared with Glasgow outcome scale (GOS) score.
Results
The mean volume ratio of residual hematoma was 59.5% and 17.6% immediately after aspiration and after continuous drainage for an average of 2.3 days, respectively. Delayed aspiration group showed significantly lower residual volume ratio immediately after aspiration. However, there was no significant difference in the residual volume ratio after continuous drainage. The favorable outcome of 1-month GOS 4 or 5 was significantly better in the group with delayed aspiration after more than 7 days (p = 0.029), despite no significant difference in postoperative 6-months GOS score. A factor which has significant correlation with postoperative 6-months favorable outcome was the final hematoma volume ratio after drainage (p = 0.028).
Conclusion
There is no difference in final residual volume of hematoma or 6-months neurologic outcome according to the surgical timing of hematoma aspiration. The only factor affecting the postoperative 6-months neurologic outcome is the final volume of remaining hematoma after drainage.
INTRODUCTION
Intracerebral hemorrhage (ICH) accounts for 8–30% of the total stroke, although there is a difference by race. ICH is a serious disease with mortality and morbidity ranging from two to six times higher than ischemic stroke.5) The most common cause is arterial hypertension and about 60% of hypertensive ICH occurs in basal ganglia. There is still controversy about the optical treatment of ICH. For the surgical indications, surgical removal is not necessary if the amount of hemorrhage is small with alert consciousness and minimal neurologic deficits; however, moribund patients with extensive hemorrhage require surgical removal. In the cases of the rapidly progressing deterioration of consciousness, urgent surgical removal wound be beneficial for lifesaving.28) There is much controversy on when and how the removal of hematoma should be performed in the patients who are initially non-comatose with neurologic deficits. Rapid removal of hematoma would improve perfusion of the compressed brain parenchyma, relieve intracranial hypertension, and eliminate the blood breakdown product, thereby minimize secondary brain edema and its neurotoxicity.5)21)28) However, open surgery through craniotomy could cause trauma to the surrounding brain structures, and neurologic deficits might remain stationary or even worsen.16)17) For this reason, initially non-comatose patients often do not undergo immediate surgery. Instead, aspiration using a non-invasive method such as stereotactic frame or neuronavigation system has been usually performed in the subacute stage. The patients who underwent non-invasive hematoma aspiration show low short-term mortality, and have the advantage of being able to early rehabilitation because of the rapid recovery.34) However, there are few studies on when non-invasive hematoma aspiration is most helpful. The aim of this study was to identify the optimal timing of stereotactic aspiration and analyze the factors affecting the clinical outcome.
MATERIALS AND METHODS
From January 2005 to December 2015, 202 patients underwent surgical treatment for spontaneous ICH in our institution, including 74 patients with decompressive craniectomy, 28 patients with craniotomy, 14 patients with endoscopic surgery, and 84 patients with minimal invasive aspiration. Of the 84 patients who underwent minimal invasive aspiration, 81 patients were included in this retrospective study, except three patients with hemorrhage resulted from brain abscess, aneurysmal rupture, and located in cerebellum, respectively. Minimal invasive hematoma aspiration was primarily considered for the following patients: spontaneous ICH without brain stem involvement, Glasgow coma scale (GCS) ≥ 5, hematoma volume ≥ 25 mL with neurological signs and symptoms, no surgical contraindications including hemorrhagic diathesis or serious uncontrollable medical illness, no underlying diseases including vascular abnormality and brain tumor. It was also performed when the patient or family refused an invasive procedure. Aspiration was recommended to be performed as early as possible, but it was performed on median 2 days after onset considering that aspiration and drainage may not be easily done because the hematoma would not dissolve in early stage. In the cases of hematoma with too much amount, aspiration was performed earlier with thrombolysis using urokinase.
Operative technique
Minimal invasive aspiration was usually performed under local anesthesia, but patients requiring endotracheal intubation due to poor consciousness received surgery under general anesthesia. Localization of hematoma was performed by using Cosman-Robers-Wells (Radionics, Burlington, MA, USA) stereotactic frame under computed tomography (CT)-guidance until 2011 and neuronavigation system (Stealth Station, Medtronic, Broomfield, CO, USA) was used as a frameless technique in some patients since 2012. In the cases using stereotactic head frame, head frame was fixed to the patient under local anesthesia and axial CT scan was performed with 5-mm slice thickness. X, Y, and Z coordinates of target point relative to the stereotactic frame were calculated and approached through ipsilateral Kocher's point or the nearest non-eloquent cortex from hematoma. Neuronavigation was used after co-registration by setting a reference point with an error range of less than 2 mm. One to three aspiration tracks were used according to the shape and size of the hematoma. After insertion of soft ventricular catheter with 2-mm inner diameter with rigid metal guidance cannula, the metal cannula was removed, and hematoma was aspirated using a syringe applying negative pressure manually. The hematoma was aspirated gently with as little negative pressure as possible, so that the syringe scale did not exceed 2–3 cm. The catheter was connected to a closed drainage system and postoperative CT scan was performed immediately after aspiration. Thrombolytic therapy with urokinase irrigation was performed if the remaining hematoma volume was more than 15 mL. Urokinase 5,000 IU was mixed with 1 mL of sterile saline every 6 hours for 1–3 days, the catheter was clamped for 1 hour, and opened again to release it naturally.
Pre- and post-operative evaluation
Patients stayed in the intensive care unit while maintaining catheter drainage and until the neurologic status stabilized. Bedside rehabilitation therapy was usually performed as early as possible within 3 days of onset. The symptoms and GCS score at the time of admission were recorded. The neurologic outcome was compared with Glasgow outcome scale (GOS) score after 1 month and 6 months after hematoma aspiration. GOS 4 and 5 were classified as favorable outcomes. Volume of hematoma was calculated using the A × B × C / 2 formula based on CT scan at admission, just before aspiration, immediately after aspiration, and after drainage, as follows.10)13)
Volume of hemorrhage = A × B × C × slices / hemorrhage shape (A = length, B = width, C = slice width)
A is the longest diameter in the axial CT image, B is the diameter perpendicular to A in the same slice, and C is the slice width, 1 for 75% to 100% of the slice measured A and B, 0.5 for 25% to 75%, and 0 for 25% or less. Patients were classified by surgical timing from onset and volume of residual hematoma after aspiration. Surgical timing was divided into < 1 day, 1–3 days, 3–7 days, and ≥ 7 days. The volume of residual hematoma after aspiration was divided into 60% or more and less than 60% compared to the volume of hematoma before aspiration.
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 (Mineapolis Inc., Chicago, IL, USA). The characteristics and results of each patient group were compared using the λ2 test, the Student's t test, and the Mann-Whitney U test.
RESULTS
The mean age of the 81 patients included in this study was 63 years (range, 24–91), including 50 men (62%), and 31 women (38%). The location of hematoma was basal ganglia in 57 patients (70.4%), thalamus in one patient (1.2%), and subcortical in 23 patients (28.6%). The location of subcortical hemorrhage was frontal in 10 patients, temporoparietal in five patients, parietal in four patients, frontoparietal in two patients, and paretooccipital/temporal in one patient each. The side of hematoma was left side in 42 patients (52%) and right side in 39 patients (48%). Intraventricular hemorrhage was found in 24 patients (29.6%). As a past history, 47 patients (58.0%) had hypertension, 15 patients (18.5%) had diabetes mellitus, seven patients (8.6%) had hepatic or renal disease, six patients (7.4%) had history of stroke, five patients (6.2%) had heart disease, three patients (3.7%) had alcohol abuse, three patients (3.7%) with anemia, and one patient (1.2%) with malignancy and platelet dysfunction, respectively. Initial presentations were motor weakness (36 patients, 44.4%), mental change (26 patients, 32.1%), headache (six patients, 7.4%), aphasia (six patients, 7.4%), dysarthria (three patients, 3.7%), drowsiness (two patients, 2.5%), seizure (one patient, 1.2%), and visual disturbance (one patient, 1.2%).
For minimal invasive aspiration, frame-based CT-guidance aspiration was used in 64 patients (79%), frameless aspiration using neuronavigation in 14 patients (17%), and three patients (4%) with hemorrhage located in the subcortical area were operated manually without an assistance. In five patients, the hematoma volume was increased at CT scan before aspiration than performed at admission, and the degree of volume increase was 104–323%. The mean volume of hematoma before aspiration was 53.5 mL (range, 15.8–184.2), and the median GCS score was 13 (range, 5–15). The mean interval time from symptom onset to hematoma aspiration was 65 hours (range, 8–666). Intraoperative thrombolytic therapy using urokinase was performed in nine patients (11.1%), mostly between 2005 and 2009. The mean volume ratio of residual hematoma on CT scan immediately after aspiration was 59.5% (range, 1.6–448). In five patients (6.2%), the volume of hematoma was increased to mean 192% (range, 103–448) after aspiration. After aspiration, hematoma drainage was maintained for an average of 2.3 days (range, 0–7), and the mean drained amount was 31.7 mL (range, 0–150). CT scan just after drainage was performed in 67 patients, and the mean volume ratio of residual hematoma was 17.6% (range, 0–72.2).
Urokinase irrigation was performed in 47 patients (58%). At the evaluation of neurologic outcome after 1 month of aspiration, five patients (6.1%) died within 1 month after aspiration (GOS 1), 24 patients (29.6%) were vegetative (GOS 2), 37 patients (45.7%) were dependent with severe disability (GOS 3), nine patients (11.1%) were independent with moderate disability (GOS 4), and six patients (7.4%) showed good recovery (GOS 5). GOS assessment after 6 month of aspiration was available in 64 patients. The number of patients in each GOS score was five patients (7.7%, GOS 1), five patients (7.7%, GOS 2), 34 patients (52.3%, GOS 3), 14 patients (21.5%, GOS 4), and six patients (9.2%, GOS 5). There was on mortality after 30 days.
Four patients underwent additional surgical treatment after stereotactic aspiration. One patient with increased hematoma immediately after aspiration and one patient who had failed to adequately remove the hematoma and could not resolve the mass effect underwent decompressive craniectomy. Another two patients underwent craniotomy and hematoma evacuation due to the increased volume of hematoma on follow-up CT scan during drainage. There was no infection associated with minimal invasive hematoma aspiration.
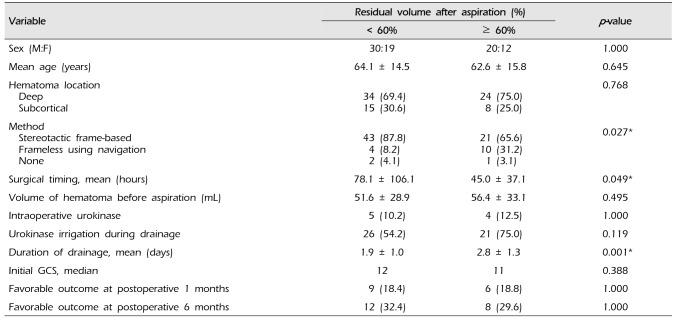
Patients were classified according to surgical timing: 27 patients (33.3%), < 1 day; 33 patients (40.7%), 1–3 days; 16 patients (19.8%), 3–7 days; five patients (6.2%), ≥ 7 days. Table 1 shows the characteristics of the patient group and the volume of hematoma calculated based on the CT scan. There was no statistically significant difference in the age, sex, hematoma location and side, but hematoma tended to be located in subcortical area and right side in delayed aspiration group. Patients with IVH all underwent aspiration within 7 days (p = 0.046). As aspiration was performed in delayed fashion, the median GCS score was statistically significantly higher (p = 0.011). This seems to be related to non-dominant side and subcortical location of hematoma. When compared the volume of hematoma according to surgical timing, there was no difference in volume before aspiration. The residual volume ratio of the CT scan immediately after aspiration was significantly lower with delayed aspiration. However, there was no significant difference in the residual volume ratio of the last CT scan performed after continuous drainage. Fig. 1 describes the volume ratio before aspiration, after aspiration, and after drainage according to surgical timing. Except of the five patients who underwent aspiration after 7 days with median initial GCS score of 15, the earlier the aspiration was performed, the shorter the intensive care unit stay period was, without statistical significance (7.1 ± 5.7 vs. 8.6 ± 7.6 vs. 11.4 ± 7.8 vs. 8.2 ± 8.7 days, p = 0.162).
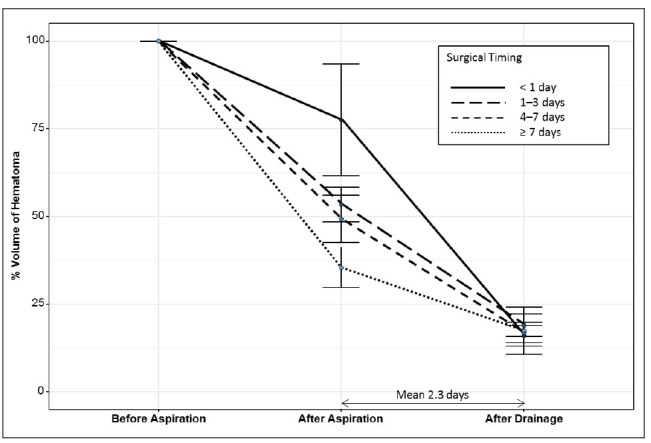
The volume ratio before aspiration, after aspiration, and after drainage according to surgical timing. When compared the volume of hematoma according to surgical timing, there is no difference in volume before aspiration. However, the residual volume ration of the CT scan immediately after aspiration is significantly lower with delayed aspiration, without significant difference in the residual volume ratio of the last CT scan performed after drainage. CT = computed tomography.
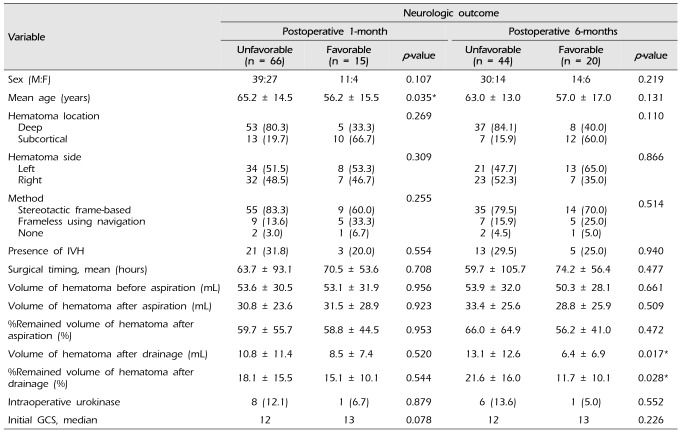
Table 2 shows the neurologic outcome with GOS score after 1 and 6 months of aspiration according to surgical timing. The favorable outcome of 1-month GOS 4 or 5 was significantly better in the group with delayed aspiration after more than 7 days (p = 0.029) (Fig. 2). This is associated with that delayed aspiration was usually performed in a neurologically stable group with a high GCS score. However, there was no significant difference in postoperative 6-months GOS score between the four patient groups.
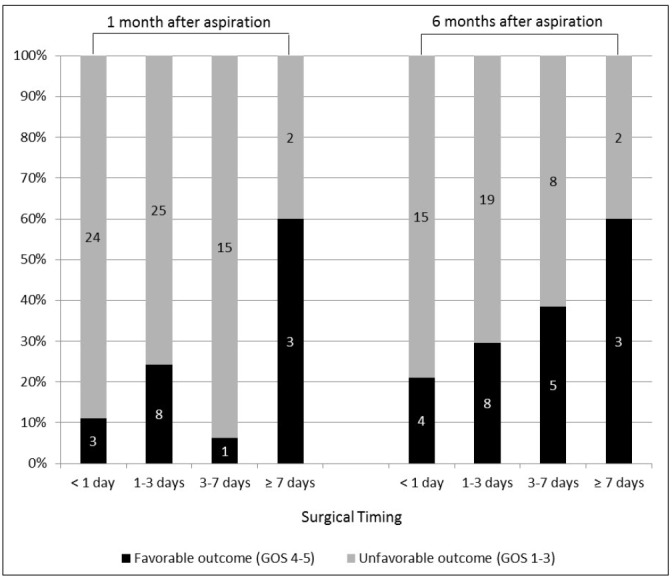
The ratio of favorable (GOS 4–5) and unfavorable outcome (GOS 1–3) after 1 and 6 months after aspiration according to surgical timing. The favorable outcome of 1–month GOS 4 or 5 is significantly better in the group with delayed aspiration after more than 7 days (p = 0.029). There is no significant difference in postoperative 6-months GOS score between the four patient groups (p = 0.363). GOS = Glasgow outcome scale.
Table 3 is an analysis of the factors affecting residual volume after aspiration and the difference in subsequent neurologic outcomes. Factors including sex, age, hematoma location, volume of hematoma, urokinase irrigation during drainage, and initial GCS did not affect the residual volume after aspiration. However, the stereotactic frame was used at a higher rate in the patient group with residual volume less than 60%, and neuronavigation use rate was higher in the group with residual volume more than 60% (p = 0.027). In addition, we found that as the stereotactic aspiration was performed later from onset, more hematoma volume was removed (78.1 ± 106.1 vs. 45.0 ± 37.1 hours, p = 0.049). There was no statistically significant correlation between residual volume immediately after aspiration and GOS after 1 or 6 months. Patients with residual hematoma volume ratio less than 60% had drainage for mean 1.9 days, while those over 60% had drainage for mean 2.3 days.
Table 4 shows the factors affecting the favorable outcome after 1 and 6 months of aspiration. The mean age of patients with a favorable outcome after 1 month was 56 years, which was significantly lower than the mean age of 65 years with unfavorable outcome (p = 0.035). A factor which has significant correlation with postoperative 6-months favorable outcome was the volume of final hematoma after drainage (p = 0.017) and the final hematoma volume ratio after drainage compared to initial volume (p = 0.028). There was no significant correlation between neurologic outcome and sex, age, hematoma location and side, aspiration method, presence of IVH, surgical timing, volume of hematoma before aspiration, remained volume after aspiration or initial GCS.
DISCUSSION
Stereotactic surgery was introduced as a less invasive alternative procedure in 1965.2) Since then, there have been reports of the benefits of stereotactic aspiration in primary ICH, despite limitations of an absence of a randomized trial comparing stereotactic surgery with medical therapy.8)18)24) A report of endoscopic surgery published in 1989 revealed a surgical benefit over medical therapy.1) Subsequently, thrombolytic therapy including urokinase and recombinant tissue plasminogen activator.15)23)27) and adjunctive technique such as intraoperative magnetic resonance imaging3) were added to conventional CT-guided stereotactic method. Although still questionable, stereotactic surgery for primary ICH currently has been a widely used procedure, and is performed on the assumption that early removal of hematoma would be beneficial for at least some patients. In a trial of early craniotomy or stereotactic surgery in a small number of patients, early surgical intervention resulted in lowering a 3-month morbidity in patients with supratentorial ICH.34) On the other hand, ultra-early craniotomy within 4 hours of occurrence seemed to increase risks of rebleeding and mortality.22)
Minimally invasive techniques such as stereotactic aspiration recently have been regarded as promising alternative methods to replace craniotomy in the treatment of ICH.26)29) A large number of case series have shown the usefulness of minimal invasive aspiration in patients with ICH.4)30) Hematoma aspiration not only alleviates the mass effect from hematoma but also is helpful for the prognosis of patients with ICH by minimizing brain tissue damage while maintaining homeostasis.6) Previous systematic reviews comparing craniotomy with conservative treatment reported that the odds ratio of unfavorable outcome was significantly lower after surgical evacuation.19)25) When comparing craniotomy with stereotactic aspiration, stereotactic aspiration lowered the morbidity of patients with primary ICH and showed a significantly lower risk of rebleeding than craniotomy, although there was no significant difference in complication.31)
There are various factors that affect the prognosis of surgical intervention including minimal invasive aspiration and craniotomy as a treatment for ICH, such as the degree of brain injury by surgical method, location of ICH (superficial versus deep-seated), level of consciousness at presentation, volume of hematoma, presence of IVH, and a patient's condition.11)25)
Surgical timing in craniotomy for ICH can be found in the Surgical Trial in Lobar Intracerebral Hemorrhage (STICH) II.20) The STICH II trial compared early surgery group with initial conservative treatment group. They found that early surgery did not increase mortality and disability at 6 months, and has a slight survival benefit in patients with spontaneous superficial ICH without intraventricular hemorrhage.20) Surgical timing of stereotactic aspiration was reported in 63 patients with primary ICH in basal ganglia, in 2003.14) The authors compared patients divided into groups that had been operated before and after 24 hours, and reported that early aspiration group showed a better neurologic outcome at the early recovery phase but no difference in the final outcome. The difference from our study was that the surgical timing was widely divided on a 24-hour basis and that the early phase outcome was better in the early surgical group. These differences may be due to the fact that our study included patients with more hematoma volumes and that the location of ICH included in our study varied.
ICH leads to cerebral edema formation and influx of neuroactive agents into the perihematomal brain tissue, causing secondary brain injury and worsening prognosis. Blood-brain barrier (BBB) disruption is a hallmark of brain injury caused by ICH, which includes inflammatory mediators, thrombin, hemoglobin breakdown products, oxidative stress, complements, and matrix metalloproteinases.12) Perihematomal glutamate level was found to increase BBB permeability and brain edema.7) The degree of secondary brain injury is related to the exposure time of brain to hematoma and volume of hematoma. In the experimental study measuring the perihematomal glutamate level, BBB permeability, and brain water contents in rats after ICH aspiration at 6, 12, 18, and 24 hours after onset, the group with removal of hematoma between 6 and 12 hours from bleeding showed the greatest pathophysiological reduction compared to the control group.32)
In this study, no patient underwent aspiration within 6 hours of onset, and two patients within 6–12 hours were included. One patient underwent stereotactic aspiration for hematoma of 34.1 mL after 10 hours. Remaining hematoma of 24.2 mL (76.9%) was found immediately after aspiration, but final remaining volume was 7.3 mL (21.4%) after urokinase irrigation for 2 days. The patient's GOS score was 3 in both 1 and 6 months follow-up. The other patient underwent early aspiration at 8 hours of onset because hematoma volume was increased from 15.1 mL to 19.8 mL in the follow-up CT and the hematoma rebleeding was suspected. On the CT just after aspiration, the amount of hematoma was significantly increased into 88.67 mL (448.3%). Craniectomy was required and both 1 and 6 months GOS score was 2. In our experience, it was safe to perform aspiration after at least 12 hours of onset, considering the risk of early rebleeding and the need to check the increase of hematoma volume requiring craniotomy than minimally invasive aspiration.
In this study, the volume of final hematoma after drainage was an important factor for prognosis which has significant correlation with postoperative 6-months favorable outcome. The relationship between the amount of hematoma and poor prognosis is likely to be influenced by the degree of damage to the corticospinal tract. Recently, diffusion tensor images have been used to minimize further injury of the corticospinal tract during surgical approach.9)33) Although diffusion tensor image was not included in this study because it was not performed in most of the patients, it would be useful if a correlation between preoperative corticospinal tract injury and prognosis could be identified using the diffusion tensor image in further study.
This study has some limitations. Because of the retrospective design, heterogeneous patients with various hematoma volume and locations were included. In addition, the surgical method for aspiration, and the use of intra/postoperative urokinase irrigation could not be controlled. Further prospective study including pathologic analysis of hematoma and perihematomal brain tissue would be required, to find out the relationship between the degree of brain injury according to surgical timing of ICH aspiration and clinical prognosis.
CONCLUSION
The earlier the ICH aspiration, the more volume left just after aspiration. However, after 2–3 days of drainage with or without thrombolytic agent, a similar amount of 16–19% of residual hematoma is left. There is a difference in neurologic outcome after 1 month according to surgical timing of hematoma aspiration, but it eventually becomes similar after 6 months. The only factor affecting the postoperative 6-months neurologic outcome is the final volume of remaining hematoma after drainage.
ACKNOWLEDGEMENTS
This research was supported by the Soonchunhyang University Research Fund.
Notes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.