Jugular foramen paragangliomas: preoperative transcatheter particle embolization
Article information
Abstract
Jugular foramen paragangliomas (JFP) are benign tumors of neural crest origin that are located along the temporal bone in the region of the jugular bulb and middle ear. The optimal management of these lesions includes surgical excision with or without preoperative embolization as well as stereotactic radiotherapy. The use of preoperative embolization in the treatment of JFP has shown great promise to bridge patients to surgery by diminishing complication rates and decreasing intraoperative bleeding. We present three successful polyvinyl alcohol (PVA) particle embolizations of patients presenting with symptomatic JFPs. All patients recovered completely in the short term with no bleeding during or after resection of paragangliomas and they were discharged free of their presenting symptoms. Early clinical and imaging diagnosis followed by adequate treatment including preoperative transcatheter particle embolization and surgical or radiosurgical interventions can lead to excellent outcomes.
INTRODUCTION
Jugular foramen paragangliomas (JFP) are benign slow-growing tumors derived from the glomus cells of the vegetative nervous system that are located along the temporal bone in the region of the jugular bulb and middle ear [1]. Glomus tumors are rare, seen in about 1 per 1 million patients and are markedly predisposed to present in women, mostly in the sixth to seventh decade of life [2].
In the region of jugular foramen, paragangliomas behave in an expansive and locally destructive manner, frequently causing cranial nerve damage with related complaints including dysphagia, hearing loss, pulsatile tinnitus, hoarseness, pain and cranial nerve palsies. Clinical diagnosis is usually confirmed by computed tomography (CT), magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). digital subtraction angiography (DSA) is especially useful to delineate the feeding artery of the tumor and potentially embolize tumor vasculatures prior to surgical resection. The use of selective angiography and super-selective embolizations in restricting vascular supply to JFP can have a significant pre-operative therapeutic role to reduce tumor burden, decrease intraoperative bleeding and facilitate tumor resection. Here, we present our neuroendovascular experience of three cases with symptomatic JFPs, which were successfully treated by preoperative transcatheter polyvinyl alcohol (PVA) particle embolizations.
DESCRIPTION OF CASES
Case 1
A 31-year-old woman with a history of recurrent left ear canal polyps of four years duration, presented with bloody otorrhea and pulsatile tinnitus. Brain MRI with contrast revealed an enhancing vascular mass, measuring 3×3×2.5 cm, occupying the left jugular foramen and tympanic region compatible with Fish type B glomus jugulotympanicum paraganglioma (Fig. 1) as confirmed by pathology of resected tumoral materials as detailed below. “Salt and pepper” appearance of the mass was noted and indicative of internal flow voids given the vascularity of JPF.
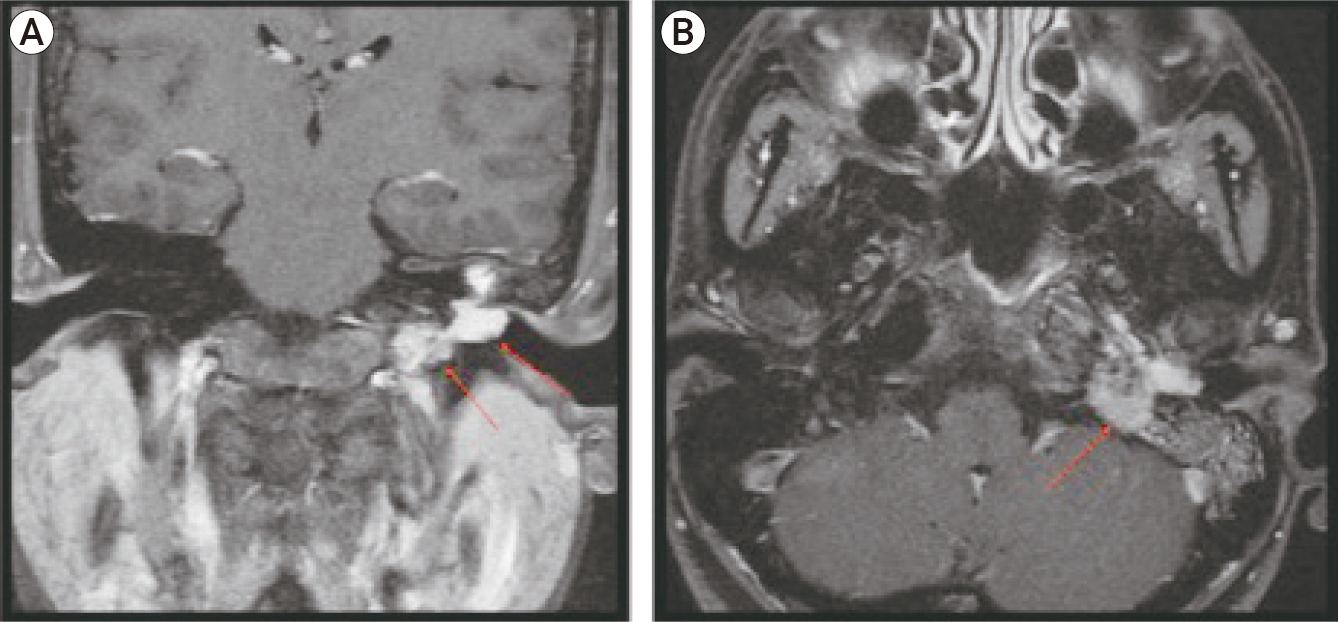
Coronal (A) and Axial (B) brain magnetic resonance images with contrast. Enhancing vascular mass (arrow) suggests glomus jugulotympanicum paraganglioma located in the left jugular foramen with complete obliteration of the tympanic cavity. Note the internal flow voids that is characterized by the “salt and pepper” appearance of the mass.
After calling timeout using universal protocol and providing moderate sedation, a 6 Fr vascular sheath (Prelude, Merit Medical Systems, Malvern, PA, USA) was placed into the right common femoral artery, accessed under sonography, using the standard Seldinger technique with maximal sterile conditions. A right common femoral angiogram was first performed to anticipate the applicability of the closure device at the end of the procedure. All angiograms were performed sequentially using an automated injector (ACIST Medical Systems, Eden Prairie, MN, USA) loaded with non-ionic contrast medium (Iodixanol 270, GE Healthcare, Chicago, IL, USA) under a flat-panel digital subtraction angiographer (Allura Xper FD20, Philips, Amsterdam, Netherlands).
Thoracic arch aortogram was performed first in 20 degrees left anterior oblique using catheter (Soft-Vu Pigtail 5 Fr/100 cm, AngioDynamics. Medical Devices, Latham NY, USA). Selective bilateral Common Carotid Artery (CCA) angiograms, Internal and External Carotid Artery (ICA & ECA) angiograms were then performed using JB1 catheter (5 Fr/100 cm, AngioDynamics. Medical Devices). A left CCA contrast injection was performed to ensure that no ECA to ophthalmic artery anastomoses exist that could compromise the patient’s vision in the event of non-target embolization. DSA angiography of the left external carotid artery prior to embolization shows intense tumoral blushes from feeding tortuous and hypertrophic jugular branch coming off the neuromeningeal trunk of the left ascending pharyngeal artery (APA) (Fig. 2). Super-selective angiograms of ascending pharyngeal artery were then performed via microcatheter system (Progreat 2.8 Fr Terumo Medical Corporation Somerset, NJ and Fathom 16 Steerable Guidewire, Boston Scientific Corporation, MA, USA) introduced coaxially via the JB 1 catheter. The microcatheter system was placed in the feeding vessels, as close as possible to the glomus tumor. Super-selective embolization of tumoral feeders coming off distal branches of APA was performed with PVA foam particles (100-300 μm, Cook Medical, Bloomington, IN, USA) mixed in a 1:1 ratio with contrast adopting a pulse pattern of injection synchronous with systole (Fig. 3A, B). If the flow to the tumor did not significantly decrease, larger PVA particle sizes (500 μm) were used until cessation of flow with no reflux of contrast along the microcatheter under real time magnification and collimation fluoroscopic modes. Post-embolization angiography revealed significant reduction of tumor blood supply and absence of tumoral blushes (Fig. 3C). Catheter and sheath systems were then retrieved in full integrity and the femoral arteriotomy was sealed with an AngioSeal 6 Fr STS closure device (Terumo, Tokyo, Japan) to expedite patient recovery and ambulation. There were no known immediate complications after the embolization except for minimal transient left facial pain.

(A) Digital subtraction angiography in an oblique lateral view of the left external carotid artery prior to embolization shows intense tumoral blushes (dark arrow) from feeding tortuous and a hypertrophic jugular branch coming off the neuromeningeal trunk of the left ascending pharyngeal artery. (B) A companion anatomical schematic is also drawn and annotated in detail.
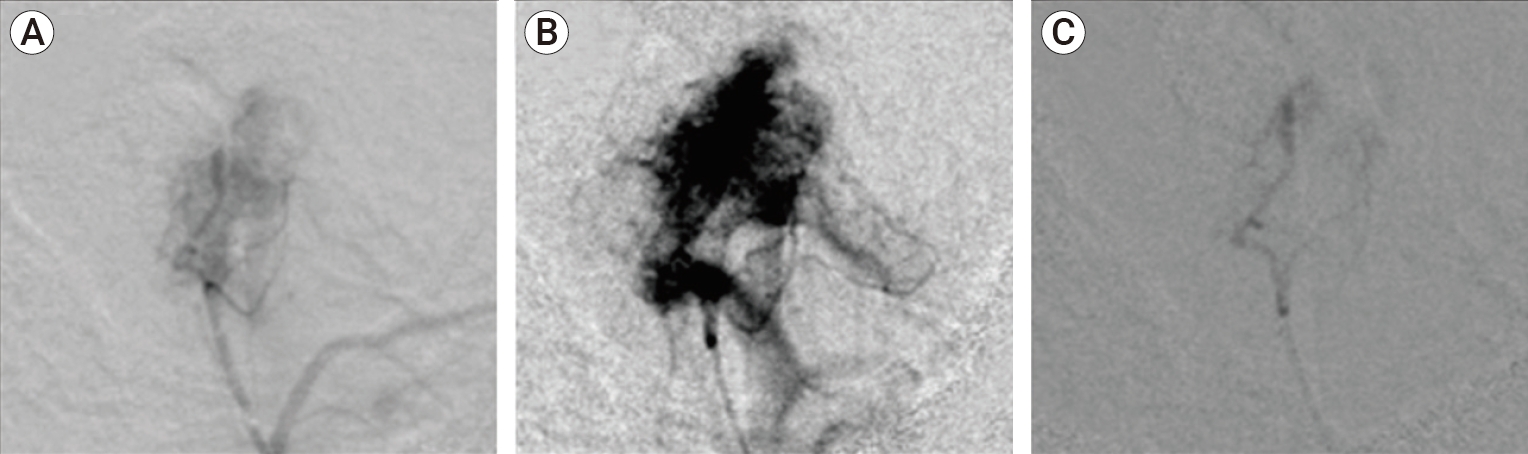
(A) Pre-embolization selective digital subtraction angiography (DSA) demonstrates tumoral hypervascular blushes within Jugular Foramen Paraganglioma fed by the tortuous and hypertrophic inferior tympanic artery and jugular branches coming off the neuromeningeal trunk of the left ascending pharyngeal artery (APA). (B) Pre-embolization Super-selective DSA in a magnified and overexposed view demonstrates very intense tumoral blushes within Jugular Foramen Paraganglioma. (C) Post-embolization superselective DSA demonstrates significant reduction in tumor vascularity, complete tumor devascularization, effacement of tumoral blushes with contrast stasis along the inferior tympanic artery and jugular branches of the left APA with no reflux into non-target vessels.
The patient was then admitted for observation unit with the intention to treat surgically within 24-48 hours post embolization. Patient was placed under general anesthesia with neurophysiologic monitoring prior to the procedure. Resection of JFP and middle ear contents was performed via infratemporal fossa approach and transmastoid repair with abdominal fat graft with subsequent implantation bone anchored hearing aids. There was no intraoperative bleeding, no immediate or late complications with a prompt duration of operation. Pathology of resected materials demonstrated nests of polyclonal sustentacular cells with thin fibrous stroma with antibodies against S100-protein, Synaptophysin (SYN), Neuron Specific Enolase (NSE), chromogranin A (CGA) on immunohistochemistry stain, consistent with paraganglioma.
Case 2
A 65-year-old woman presented with hoarseness and hearing loss of two months duration. On physical examination, she was found to have paralysis of left cranial nerves VIII, IX, and X. Head CT imaging demonstrated a moth-eaten appearance of the posterior aspect of the left petrous apex with erosion of the posterior medial wall of the left bony carotid plate (Fig. 4). Brain MRI with contrast revealed an enhancing vascular mass, measuring 4×3.5×3 cm, with “salt and pepper appearance” and flow voids consistent with Fish type C JFP (Fig. 5).

Axial (A) and Coronal (B) head CT images demonstrate moth-eaten appearance of the posterior aspect of the left petrous apex, expansion of the left jugular foramen (arrows), and erosion and dehiscence of the posterior medial wall of the left bony carotid plate compatible with paraganglioma /glomus jugulare tumor. CT, computed tomography.
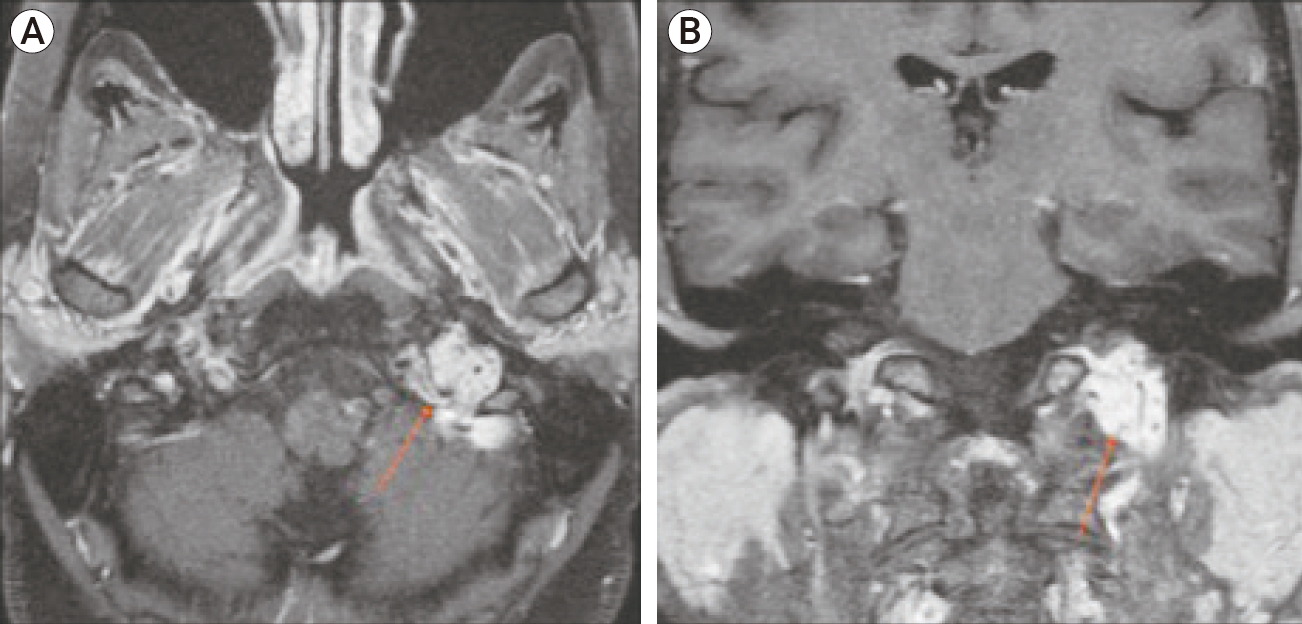
Axial (A) and Coronal (B) brain magnetic resonance images with contrast. Enhancing vascular mass (arrow) located in the left jugular foramen suggests glomus jugulare paraganglioma. Characteristic “salt and pepper” appearance of the mass is indicative of internal flow voids representing the hypervascularity of the tumor.
The endovascular approach, materials and tools were used similarly to those described in the case 1. Following embolization, selective and super-selective angiograms were performed to demonstrate complete stasis and effacement of tumoral blushes. (Fig. 6A, B). JFP surgical resection via infratemporal fossa approach and transmastoid dural tear repair with abdominal fat graft was successfully performed within 24-48 hours of embolization. There were no immediate or late complications with near complete resolution of presenting symptoms in the post-surgical resection period. Pathology of resected materials demonstrated similar microscopic and immune-histochemical findings to the case 1, consistent with paraganglioma.
Digital subtraction angiography (DSA) of the left external carotid artery. (A) DSA prior to embolization shows intense tumoral blushes (lighter arrow) from feeding arterial branches deriving from the left ascending pharyngeal artery (black arrow) as part of the external carotid circulation. (B) Super-Selective DSA in a magnified view following successful embolization demonstrates significant reduction in jugular foramen paragangliomas vascularity and blush effacement (lighter arrow).
Case 3
A 50-year-old woman with a history of pulsatile tinnitus in the left ear, presented with left sided facial numbness. Brain MRI with contrast revealed enhancing vascular mass with internal flow voids in the left jugular foramen compatible with Fish type C glomus jugulare paraganglioma (Fig. 7).

Axial (A) and coronal (B) brain MR images with contrast demonstrate enhancing vascular mass (arrow) occupying the left jugular foramen with internal flow voids in “salt and pepper” pattern suggestive of a jugular foramen paraganglioma.
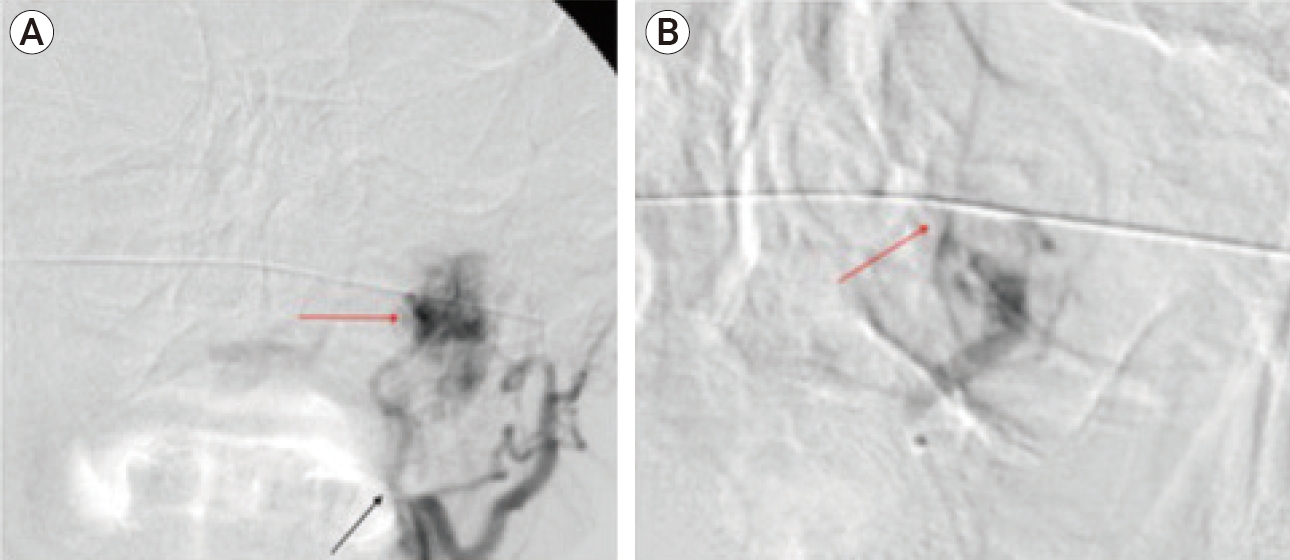
Same methods and materials were adopted similarly to the case 1, using the exact endovascular technique and tools. Embolization of the feeding jugular branches coming off the left APA resulted in significant reduction in tumor arterial flow and resolution of tumoral blushes indicating a significant suppression of tumor vascular supply (Fig. 8A, B). Resection of JFP via infratemporal transmatoid approach was performed within 24-48 hours of the embolization procedure. Pathology of resected materials demonstrated similar findings to case 1, consistent with paraganglioma.

(A) DSA of the left common carotid artery before embolization shows the feeding arterial branch from the left ascending pharyngeal artery from the external carotid artery (black arrow) with tumoral blushes (lighter arrow) projecting over the left jugular foramen region. (B) Selective DSA after successful tumoral embolization demonstrated a significant reduction in tumor vascularity and diminishment of tumoral blushes (lighter arrow) with adequate flow in the adjacent arteries. DSA, digital subtraction angiography.
There was complete resolution of the presenting symptoms for 2 years; however, a recent follow up revealed that the patient was sensitive to loud sounds with mild left tinnitus, with 2×1.8×1.7 cm recurrent left glomus tumor as reported on MRI imaging performed at outside facility. This was treated by Gamma knife radiosurgery at an outside institution with an audiogram demonstrating stable minimal sensorineural hearing loss in the left ear.
DISCUSSION
JFP are benign slow-growing tumors that are derived from the glomus cells of the parasympathetic nervous system, located along the temporal bone in the region of the jugular bulb and middle ear. JFPs are commonly classified by the Fisch classification [5]. Fisch classes A and B tumors present with minimal extension and invasion whereas Fisch class C and D tumors cause erosion, destruction, and invasion of nearby structures such as cranial nerves and bones [5,17]. Primary surgical resection of paragangliomas is challenging due to tumor hypervascularity and locations near to vital structures. Surgical tumor excision results in a reported 90% control rate of resected lesions and can be complicated by neurological consequences, which include persistent cranial nerve deficits and an overall mortality rate from 1-6% [14,22]. Stereotactic radiosurgery may carry lower morbidity than surgery with a possibility of long-term neurological symptom improvement in 40-60% of patients [10,11]. Embolization in conjunction with surgical resection is another option to treat effectively JFPs with fewer complications [6,8,12,21].
The use of pre-operative embolization in the treatment of paragangliomas has gained significant traction over the last few decades, secondary to advances in super-selective catheterization techniques and high-resolution imaging technology. The development of super-selective embolization techniques has greatly improved the efficacy of occluding specific tumoral feeders of paraganglioma tumors [18,20]. Embolization of paragangliomas prior to surgery has several advantages over the sole surgical resection approach. Several studies have demonstrated a reduction in the frequency of bleeding events and blood transfusions following surgery when pre-operative embolization is performed [1,8,12,21]. Reduction in tumor size, a common finding post-embolization, improves the likelihood of a technically successful resection and may result in a shorter operation times [11,19]. In patients with larger and more invasive jugular paragangliomas such as Fisch class C and D tumors, preoperative embolization allows for a more favorable surgical approach and increased probability of complete and uncomplicated tumor excision [6,12,19]. Angiography, apart from its role in directing embolics, also gives the surgeon a distinct advantage by conveying tumor anatomy including flow dynamics and locations of feeding vessels [19]. Pre-surgical embolization also increased quality of life metrics in glomus jugulare patients in one study, which can be attributed to the regression of clinical symptoms commonly seen post-procedure [21].
It should be noted that the use of transcatheter preoperative particle embolization in the management of jugular foramen paragangliomas is not fully curative but rather adjunctive or palliative [15,21]. Thus, the use of embolization as a primary treatment is not advised and surgery is required to ensure long-term resolution of symptoms. Following embolization, spontaneous relief of clinical symptoms is common [19,20]; however, histological evidence has demonstrated a 30% recanalization rate in tumor vessels embolized with PVA particles as early as nine days post-embolization [18]. This suggests a risk of symptom recurrence following primary embolization treatment as the tumor regains its vascularity and enlarges. In asymptomatic elderly patients with slow growing masses, primary embolization treatment can be considered in lieu of surgery as a palliative measure [21]. A “wait and scan” approach may also be adopted in these cases, especially when patients are presenting with additional underlying medical concerns [1,19].
Complications of JFP embolization can range from minor complications such as post embolization syndrome (fever, transient facial pain, discomfort) to major ones such as transient aphasia, catecholamine storm, facial skin necrosis, blindness, and stroke that can occur in the event of non-target embolization [21,24]. The presentation of fever and ear pain after embolization is common and is often an indication of a successful procedure [23]. Additionally, there are also several reports in the literature that describe the occurrence of cranial nerve deficits following glomus jugulare embolization due to the close association of tumor arterial supply with the vasa nervosa of cranial nerves [3,4,7,13]. The incidence of these cranial neuropathy complications is not well described and rates from several small retrospective studies vary greatly: 2-18% [4,14,16,23]. We did not observe any such complications in this small case series using polyvinyl alcohol particles.
The embolic chosen for the procedure plays a significant role in the modulating the risk of nerve complications. Valavanis [23] suggested that selection of the embolic should be hinge upon considerations of safety and efficacy. Use of permanent embolics such as Onyx are especially effective in that they have a very low arterial revascularization rate, but should only be administered in “safe” vessels that do not communicate with the ICA, vertebrobasilar system, or the blood supply of cranial nerves [9,23]. The potential risks of non-target embolization using Onyx may be further mitigated by utilizing balloon-assisted catheter delivery to reduce reflux and promote embolic penetration of the tumor according to one report [9]. Other agents such as PVA and other particulate embolics are preferred for use in more at-risk or “dangerous” arteries since these larger particles are unlikely to occlude nerve vasculature [3,23]. Due to their non-permanent nature, particles tend to have higher rates of revascularization [18]; thus, cranial neuropathies caused by particle embolization have increased rates of recovery when compared to the use of more permanent agents [3,4]. It can be inferred that use of smaller particles would present a higher risk of nerve deficits than the use of larger particles, attributed to the increased likelihood of occluding the small arteries that supply cranial nerves [4]. Irrespective of the embolic chosen, careful angiographic evaluation of the tumor vasculature and recognition of any variant cranial vasculature present is crucial to reduce the risk of non-target delivery and cranial nerve complications.
Our case series carries many limitations. We are presenting a very small number of patients with no control group of non-embolized resected tumors to assess the role of particle embolization in the management of jugular foramen paragangliomas in term of shortening operative time and bleeding during and after resection, as well as other variables within an adequate statistical significant model. There was no comparison to stereotactic radiotherapy or radiosurgery performed in a matching group of patients. We did not use or assess the role of other potential embolic materials including Onyx, glue, or microcoils in the preoperative therapy of JFPs.
CONCLUSIONS
Transcatheter preoperative particle embolization can be considered as a safe and effective approach in the pre-operative management of jugular foramen paragangliomas. Additional controlled trials are required to gain a stronger consensus on specific indications and the role of preoperative arterial embolization in the management of the jugular foramen paragangliomas.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
