 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 24(3); 2022 > Article |
|
Abstract
Vertebrovertebral arteriovenous fistulas (VVAVFs) are rare entities that lack consensus guidelines for their management. Our case describes the successful treatment of a traumatic VVAVF via a contralateral deconstructive endovascular approach. A 64-year-old female presented following a traumatic fall. Computed tomography angiogram highlighted a 2 cm pseudoaneurysm of the right vertebral artery (VA) with epidural contrast enhancement and a hematoma with flow voids within the epidural space. Digital subtraction angiography showed a VVAVF at C2-3 with retrograde filling of the distal right VA. Having undergone several unsuccessful passes of the proximal dissection flap in the right VA, the patient underwent a contralateral deconstructive approach with correction of the VVAVF without complication. The remaining feeding branches had occluded after 1 week. The patient made a complete recovery without neurological sequelae at 3-month follow-up.
Endovascular interventions are rapidly becoming the primary means of treatment for vertebrovertebral arteriovenous fistulas (VVAVFs). VVAVFs are rare entities that arise due development of an abnormal connection between a vertebral artery (VA) and a vertebral vein or venous plexus. If untreated, their persistence can result in immediate and late neurovascular complications. Here we describe the contralateral deconstructive repair of a traumatic VVAVF associated with a dissection flap of the parent vertebral artery, via an anterograde left VA to retrograde right VA approach. An in-depth discussion is included of the clinical literature surrounding this rare entity, the proposed pathophysiology of VVAVFs and the evidence base for management.
A 64-year-old female presented following a fall down 14 stairs. On examination, she was alert and oriented with pain-limiting weakness of her right arm and leg. The secondary trauma survey highlighted a frontal scalp laceration and right humeral fracture. The only abnormality on examination was limited range of motion in the right upper extremity secondary to the fracture.
Computed tomography angiography of the neck showed a right VA injury at the level of a C2 fracture, with a 2 cm pseudoaneurysm that appeared contiguous with prominent epidural contrast enhancement (Fig. 1A-B). Magnetic resonance imaging of the cervical spine revealed potentially unstable discoligamentous injuries at C2-3, an epidural hematoma, and prominent flow voids in the epidural space (Fig. 1C).
Catheter digital subtraction angiography (Fig. 2A-C) confirmed the presence of a pseudoaneurysm with the VVAVF causing engorgement of the internal vertebral (epidural) venous plexus centered at C2-3. The distal right VA did not fill anterograde, but did fill retrograde on left VA injection, suggesting arterial transection and/or hemodynamically significant AVF. A deconstructive approach was favored over reconstructive stenting as the patientŌĆÖs injuries required surgery. Therefore avoidance of antiplatelet therapy was a primary goal. Furthermore, there was minimal anterograde flow through the injured right VA at presentation and the left VA was robust, allowing for right VA deconstruction.
Multiple attempts to navigate a microcatheter into the right VA were unsuccessful due to a proximal dissection of the right VA. Following a multidisciplinary discussion, a contralateral approach was pursued. A microcatheter (Excelcior SL-10, 45┬░, Stryker, Tokyo, Japan) was maneuvered from the left VA across to the intracranial right VA to allow catheterization of the fistula communication where 9 coils were successfully deployed. Post-coiling angiography showed successful closure with no evidence of the fistula filling from the left VA, but angiography from the right subclavian showed persistent slow filling from the ascending cervical artery to the fistula. However, repeat angiograms one week and three months later revealed these remaining feeding branches to have occluded and there was no residual fistula (Fig. 2D-F). The patient made a complete recovery, returning to her premorbid modified Rankin scale of 1 at 3 months follow up. Informed consent was obtained from the patient for the procedures described.
We outline the endovascular treatment of a traumatic VVAVF via a contralateral deconstructive approach with coil embolization.
A VVAVF is an abnormal connection between a VA and a vertebral vein. A plexus of veins surrounds the VA with the transverse canal rostrally to C6 where they join to form a single vertebral vein [2]. The transverse vertebral plexus anastomoses in segmental fashion with ventral longitudinal veins in the internal vertebral venous plexus, the network of epidural veins in the spinal canal [3]. The pressure within the venous plexus is low and can even be negative when erect [5]. As such, arterial bleeding from the VA is in immediate proximity to these veins and can lead to the development of an AVF. Clinical features include venous hypertension, vertebrobasilar insufficiency through steal phenomenon, thrombosis, or haemorrhage. Up to 30% of VVAVFs are asymptomatic and discovered incidentally on angiography [2].
VVAVFs can be congenital (referred to as vascular malformations) or acquired. Congenital vascular malformations of the vertebral artery are persistent embryonic AV shunts and are frequently asymptomatic [2]. VVAVFs have been described in patients with Neurofibromatosis type 1 [1]. When acquired, they may result from trauma to the cervical region (e.g. whiplash, stab wound), after iatrogenic injury (e.g. catheter puncture or spinal surgery) or can occur spontaneously [2].
A systematic review of 280 acquired VVAVF cases and found their cause to be iatrogenic (n=68; 24%), spontaneous (n=136; 49%), or traumatic (n=76; 27%) [1]. Traumatic VVAVFs were more often found in young males, while spontaneous VVAVFs were more prevalent amongst young females, likely due to their increased prevalence of connective tissue disorders within the latter group [1]. The most common location of traumatic VVAVFs within these cases was at C1 (19% of cases) [1]. The greater occurrence of VVAVFs at the C1 level has been hypothesised to be the result of increased strain on this vessel segment due to the greater range of physiologic movement at this location [1,4]. However, there are a lack of in vivo models to test this hypothesis.
A distinction between ŌĆśupperŌĆÖ and ŌĆślowerŌĆÖ cervical lesions has been described in the literature [2]. Upper VVAVFs are associated with C1-C2 from the proatlantal system. Venous drainage of the upper VVAVFs uses the suboccipital and vertebral network [2]. Upper lesions are mostly ŌĆśhigh-flowŌĆÖ lesions and more often require therapeutic intervention. Lower lesions are regarded as ŌĆśslow-flowŌĆÖ and may not require intervention if asymptomatic. The lower group primarily drains into the epidural space [2].
The most common clinical signs associated with VVAVFs are bruits, tinnitus (due to retrograde venous drainage in the jugular vein and skull base venous sinuses near the cochlea), and/or weakness related to radiculopathy or myelopathy [1]. Steal phenomenon can also occur resulting in symptoms of brainstem ischemia such as vertigo and diplopia [1]. In our case, the right-sided weakness noted on presentation was the result of pain secondary to trauma and not neurological damage.
The management of VVAVFs is based on their etiology, symptomatology and institutional expertise. Endovascular interventions are now favored given their safety profile and relatively shorter recovery time [1,4]. Open surgeries are still used where endovascular techniques fail and/or in cases of complex VVAVFs [4]. Optimal treatment consists of achieving complete occlusion of the fistula while maintaining patency of the VAs [2]. In some cases, spontaneous occlusion/resolution of VVAVFs has been reported [1]. These are typically iatrogenic (post-catheterisation) ŌĆśslow-flowŌĆÖ lesions. Long-term outcomes of untreated fistulas are poorly documented, and both occlusion and deterioration can occur.
Approaches to VVAVF correction are categorized as ŌĆśconstructiveŌĆÖ or ŌĆśdeconstructiveŌĆÖ [1,2,4]. Constructive approaches involve maintenance of both VAs and obliteration of only the fistula. Deconstructive management involves sacrifice of the feeding VA, requiring clear patency and function of the contralateral VA [1,4]. Various endovascular techniques can be used to maintain both VAs. Balloons can be deployed within the VA across the abnormal arteriovenous connections allowing for the placement of coils. Balloons are easy to manipulate, being inflated, deflated and moved to achieve maximal intra-procedure occlusion; however, if abnormal connections are not adequately embolized there is the risk of recurrence with deflation. Although covered stent reconstruction is also a successfully utilised technique, in rare cases, there may be complications related to stent migration, kinking, and endovascular leakage. Stents also necessitate the use of antiplatelet agents, which may increase bleeding risk [1].
In our case, the presence of an ipsilateral VA dissection necessitated deconstructive endovascular management via a contralateral approach. Surgical (non-endovascular) treatment should be reserved for unusual cases such as anatomical variations preventing catheterization, congenital malformations or those secondary to prior treatment and fistulas associated with a large pseudoaneurysm [1,2,4].
VVAVFs are rare vascular entities that may result after cervical trauma. The evolution of endovascular techniques and their widening indications has led to non-surgical management being the preferred modality of treatment. We have outlined a case of a contralateral deconstructive VVAVF correction through an endovascular approach, with successful resolution of the VVAVF and a full clinical recovery.
Fig.┬Ā1.
Non-invasive preoperative imaging. CT angiography of the neck in axial plane (A) and curved planar reformation along the right cervical vertebral artery (B) showed an asymmetrical fracture at the right pedicle and left pars interarticularis of C2 (bidirectional arrow), with extension to the right transverse process and spinous process (white arrowheads); right vertebral artery pseudoaneurysm insinuating into fracture lines (asterisk); and prominent epidural contrast enhancement (curved arrows). MRI of the cervical spine on a right parasagittal short TI inversion recovery (STIR) image (C) showed prevertebral and paraspinal fluid, discoligamentous complex injury through the C2-3 intervertebral level (outlined arrows), circumferential epidural hematoma (star), and flow voids in the pseudoaneurysm extending into the epidural space (asterisks). CT, computed tomography; MRI, magnetic resonance imaging
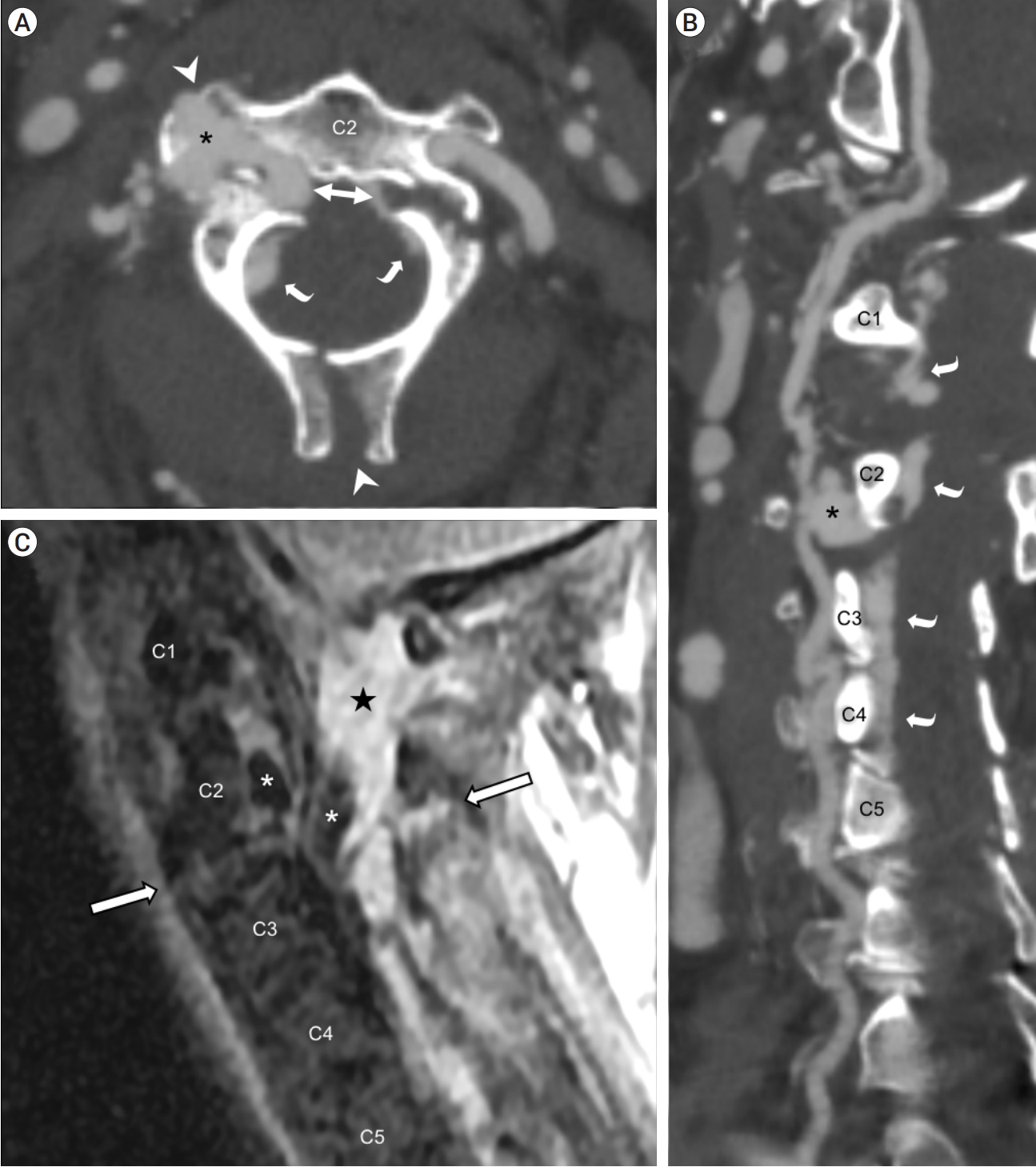
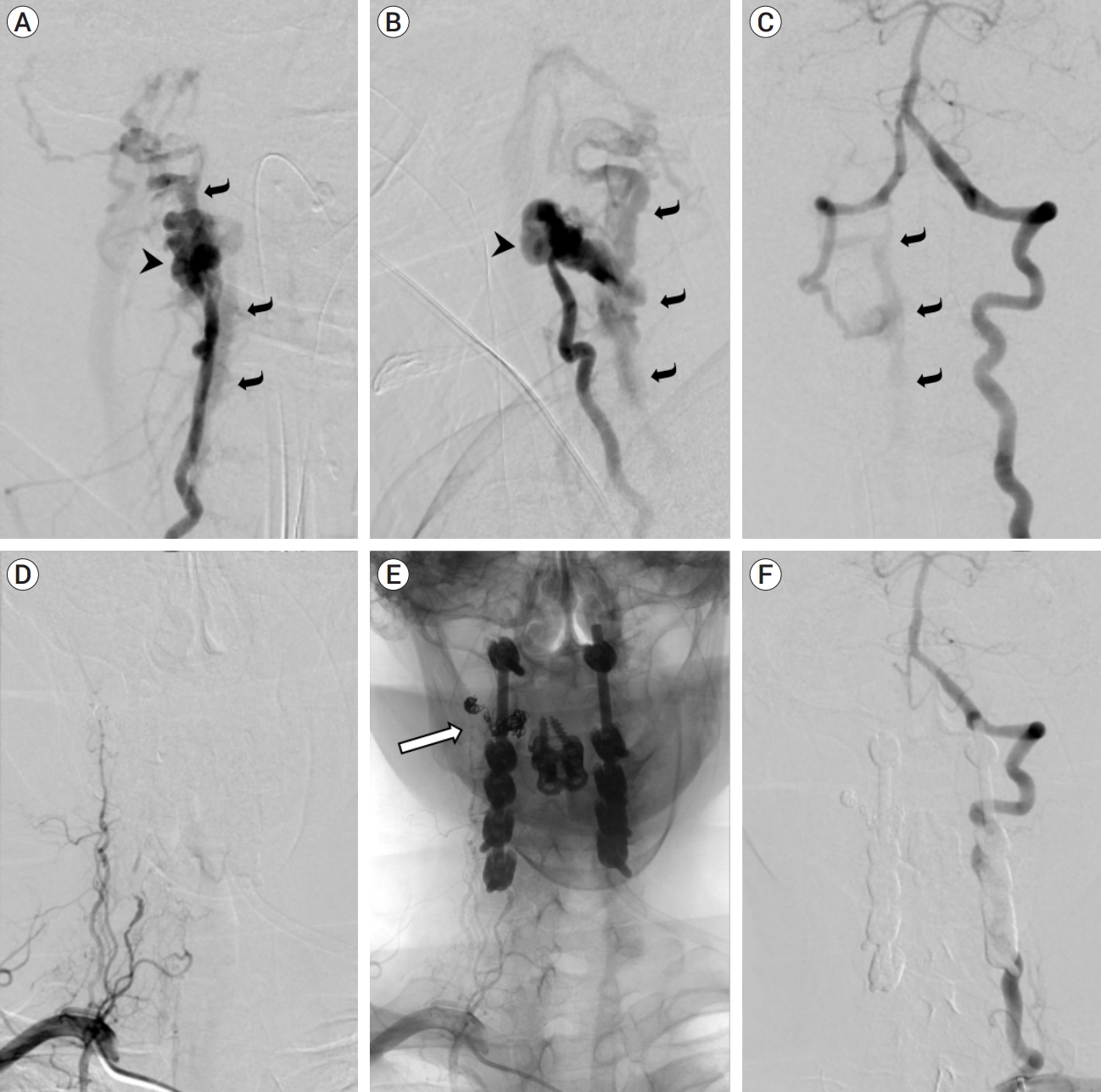
Fig.┬Ā2.
Catheter angiography before (A-C) and after (D-F) endovascular treatment and spinal fixation. Right vertebral artery injection in right anterior oblique (A) and lateral (B) projections showed pseudoaneurysmal outpouching (arrowhead) surrounding the distal V2 (intraforaminal) segment, no distal arterial opacification, and early filling of the engorged internal vertebral venous plexus and other venous structures cranial and caudal to the level of injury (curved arrows), indicating a vertebrovertebral arteriovenous fistula (VVAVF). Left vertebral artery injection in the anteroposterior projection (C) showed retrograde filling of the distal right vertebral artery and the VVAVF (curved arrows). Follow-up angiogram after coil embolization of the pseudoaneurysm and fistulous connection showed no filling of the entire right vertebral artery on right subclavian artery injection (D, subtracted image; E, unsubtracted image showing coils [outlined arrow] and spinal hardware) and on left vertebral artery injection (F). No early venous filling was shown to suggest residual fistula.
REFERENCES
1. Aljobeh A, Sorenson TJ, Bortolotti C, Cloft H, Lanzino G. Vertebral arteriovenous fistula: a review article. World Neurosurg. 2019 Feb;122:e1388-97.


2. Berenstein A, Lasjaunias P, ter Brugge KG. Vertebro-vertebral arteriovenous fistulae. In: Berenstein A, Lasjaunias P, Brugge KG, editors. Surgical Neuroangiography. New York: Springer-Verlag; 2004. p. 743-51.
3. Groen RJ, Groenewegen HJ, van Alphen HA, Hoogland PV. Morphology of the human internal vertebral venous plexus: a cadaver study after intravenous araldite CY 221 injection. Anat Rec. 1997 Oct;249(2):285-94.


- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 2,174 View
- 47 Download
- ORCID iDs
-
Sathiji K. Nageshwaran

https://orcid.org/0000-0003-1726-5374 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



