 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(1); 2023 > Article |
|
Abstract
The prevalence of aneurysm formation in adults with Moyamoya disease (MMD) is higher than that in the general population. The treatment strategy is often individualized based on the patient’s disease characteristics. A 22-year-old man was diagnosed with MMD after presenting a small thalamic intracerebral and subarachnoid hemorrhage in the quadrigeminal cistern. Cerebral angiography revealed a small aneurysm (2.42 mm) in the left anterior choroidal artery. Since the hemodynamics in the left hemisphere was compromised, an indirect bypass surgery was performed. The patient’s condition deteriorated postoperatively because of poor perfusion of the internal carotid artery, and massive hydration was required. During neurocritical care, the aneurysm increased in size (5.33 mm). An observation strategy was adopted because of the distal aneurysmal location and the high risk involved. Subsequently, the patient recovered, and newly developed collateral flow appeared from the external carotid artery. Additionally, a dramatic size reduction of the aneurysm (1.51 mm) was noticed. Our case suggests that MMD-related dissecting aneurysms on a distal cerebral artery, which present a high risk of embolization, could be managed by indirectly reducing the hemodynamic burden. Massive hydration in such cases should be avoided or balanced to avoid the risk of rapid growth and aneurysm rupture.
The prevalence of aneurysm formation in adult patients with Moyamoya disease (MMD) is approximately 14%, considerably higher than that in the general population (2-3%) [1,9]. In MMD patients, progressive occlusion of the internal carotid artery (ICA) alters the hemodynamics in other arteries, promoting compensatory dilation and increased blood flow through the collateral vessels. These alterations often lead to the formation and rupture of intracranial aneurysms [4,8]. However, the pathogenesis of MMD-related aneurysms is highly complex and can be affected by several factors, including patient age, vascular architecture, and anatomic location of the aneurysm [3]. Therefore, the treatment strategy has not been standardized and should be individualized based on a patient’s disease characteristics.
Herein, we describe a patient who presented an MMD-related aneurysm in the left anterior choroidal artery (AChA) and underwent indirect bypass surgery to decrease the hemodynamic burden on the aneurysm. Interestingly, the distal AChA aneurysm showed fluctuating changes in size during the postoperative hydration treatment. To the best of our knowledge, similar variations have never been reported.
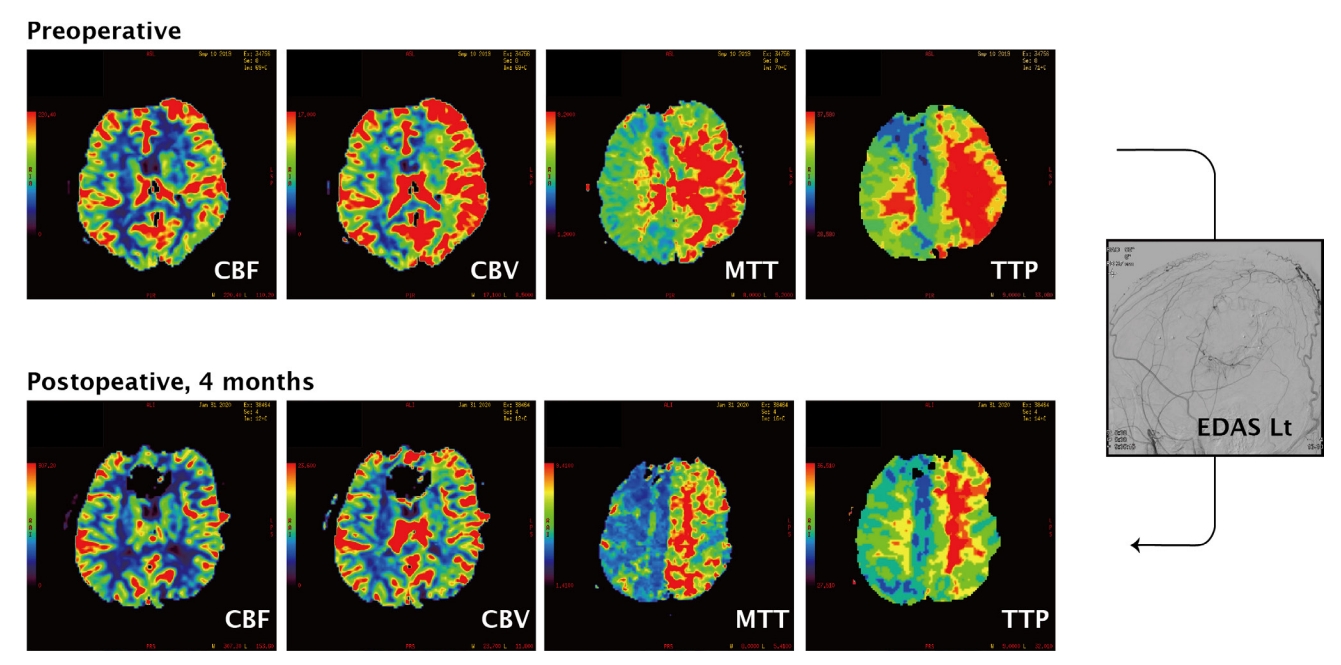
A 22-year-old male soldier was transferred to the emergency room of our hospital after experiencing a sudden headache and a brief loss of consciousness. On arrival, he was alert and presented no neurological deficits. An initial brain computed tomography (CT) revealed a small subarachnoid hemorrhage in the left quadrigeminal cistern (Fig. 1). After undergoing cerebral angiography and perfusion studies (Figs. 2 and 3), he was diagnosed with bilateral MMD with moderate-to-severe hemodynamic compromise in the left hemisphere. In addition, a 2.42-mm saccular aneurysm was identified on the plexal segment of the left AChA during his left ICA angiography. Since the hemorrhage is located far from the aneurysm on brain CT angiography, we regarded an aneurysm as unruptured. Initially, we decided not to intervene on the MMD-related aneurysm because embolization of the plexal segment of the AChA has a higher risk of complications than rupture. During the observation period (about 2 weeks), the patient experienced two different tonic-clonic seizure-like episodes, attributed to hemodynamic insufficiency of the cortex. Therefore, indirect bypass surgery by encephalo-duro-arterio-synangiosis (EDAS) was performed three weeks after symptoms onset. A direct bypass was planned initially, although it could not be performed because of the poor status of the recipient M4 arteries. Postoperatively, the patient was alert and did not present any neurological symptoms or signs. However, his clinical conditions worsened, and serial CT revealed delayed postoperative epidural and subdural hematomas. The left hemisphere was compressed without expansion due to the poor perfusion status associated with MMD and mass effect from delayed hematomas. Therefore, 2-3 L of normal saline and 0.5-1 L of a starch solution per day were perfused for hydration. Despite this robust protocol, the patient exhibited severe drowsiness and right hemiparesis (grade II), with fluctuating severity according to the hydration status. Therefore, surgical evacuation of the epidural and subdural hematoma was performed six days after EDAS. However, after second surgery, patients did not get improved. Because he showed neurological fluctuations according to the volume and blood pressure status, cerebral perfusion was maintained with 2 L of normal saline and 0.5 L of starch solution. A cerebral angiography was conducted on post-EDAS day 17. In the left common carotid artery angiogram, the AChA aneurysm was remarkably enlarged, with a maximum diameter of 5.33 mm. We adopted a conservative observation strategy again for the following reasons: (1) failure in coil embolization of the aneurysm would have trapped the plexal segment of the AChA because of its distal location, possibly leading to severe complications; (2) procedures such as microcatheter engagement through the small AChA may have caused a decrease in deep cerebral perfusion since the collateral flows originated in the proximal and distal AChA; and (3) induction of general anesthesia itself implicated a high risk of hypotensive events, known to be related to the clinical deterioration of MMD patients. Fortunately, the patient recovered three weeks after EDAS surgery, and his strength increased. Hydration was gradually tapered, and an angiogram on post-EDAS day 35 revealed a dramatic reduction in the size of the aneurysm (1.51 mm; Fig. 2). New indirect external carotid artery collateral flows developed, and we expected a decrease of the hemodynamic burden on the ICA branches (including the AChA). The hemodynamic status of the left hemisphere (especially the cortical area) significantly improved on the perfusion images after surgery (Fig. 3).
To date, there is no standardized treatment for patients with MMD-associated intracranial aneurysms. Herein, we report the successful case of a patient with an MMD-associated aneurysm treated via indirect bypass surgery.
MMD is characterized by progressive stenosis or occlusion of the distal ICA and angiogenesis of collateral vessels in proximity to the steno-occlusion. The altered hemodynamics may lead to the formation of intracranial aneurysms; its prevalence in patients with MMD is therefore considerably higher than that in the general population [1,9]. Recently, Kim et al. classified MMD-associated aneurysms into true hemodynamic aneurysms and dissecting or pseudoaneurysms based on their location and characteristics [5]. True hemodynamic aneurysms were defined as occurring along the healthy patent vessels comprising the circle of Willis, which are hemodynamically burdened to supply the brain tissue affected by MMD adequately. In patients with unilateral MMD, aneurysms of the contralateral anterior communicating artery may occur because of increased blood flow on the anterior communicating arterial channels. In contrast, dissecting or pseudoaneurysms are frequently observed in the distal basal collateral vessels such as the distal anterior choroidal or lenticulostriate arteries. Angiogenesis on the basal vessels occurs in MMD because of the chronic ischemic status of the brain, and pseudoaneurysms may develop because of microstructural pathological changes or dissection of the small vessel wall [1,5].
In our case, the AChA aneurysm could be classified as a dissecting aneurysm or pseudoaneurysm based on its relationship with the parent artery and morphological changes over time. Dissecting pseudoaneurysms also have a higher risk of rupture and should therefore be treated, if possible [6]. However, in our experience, embolization of the aneurysm involves a high risk of cerebral infarction, and the approach route is challenging. Therefore, we attempted to reduce the hemodynamic burden on the basal Moyamoya vessels with a bypass surgery. According to the literature, most physicians attempt to treat this type of aneurysm using endovascular approaches [2,6,7]. Kim et al. attempted to treat two cases of dissecting pseudoaneurysms by glue embolization, resulting in one success and one failure. In the unsuccessful case, the patient was monitored for three months, and the aneurysm regressed spontaneously [5]. Based on the case presented herein and in the previous reports, reduction of the hemodynamic burden on the aneurysm’s parent artery via bypass surgery or short-term close observation could be an alternative treatment strategy for MMD-related dissecting pseudoaneurysms [10-12].
Interestingly, we observed dynamic size changes related to the hemodynamics of the parent artery (diameter: from 2.42 mm to 5.33 mm, and finally to 1.51 mm). Fortunately, the aneurysm did not rupture despite the sudden increase in diameter (220%), and it regressed after the formation of indirect cortical collateral vessels on the operated side. However, the aneurysm carried a risk of rupture during the massive post-surgical hydration. Physicians must be aware that excessive hydration should be avoided in patients with MMD-associated intracranial aneurysms, especially when associated with dissecting or pseudoaneurysms.
The treatment of MMD-related aneurysms can be challenging and should be individualized based on a patient’s hemodynamic status. When the aneurysm is too deeply seated to be treated directly and is highly at risk of accompanying complications, a surgical bypass could be a viable option to decrease the hemodynamic burden on the parent artery. Massive hydration in cases of MMD-related aneurysms should be avoided or balanced because of the risk of rapid growth and aneurysm rupture.
Fig. 1.
Serial computed tomography (CT) images show from initial to Postoperative day 14 - Preoperative; Small amount of subarachnoid hemorrhage on the left quadrigeminal cistern on initial CT, Postoperative day 2; Clinical course got worsened (motor aphasia) and CT revealed the delayed postoperative hematoma on the epidural and subdural spaces, Postoperative day 6; Hematoma evacuation was performed due to the deep drowsy mentality with right hemiparesis (grade II). EDAS, encephalo-duro-arterio-synangiosis
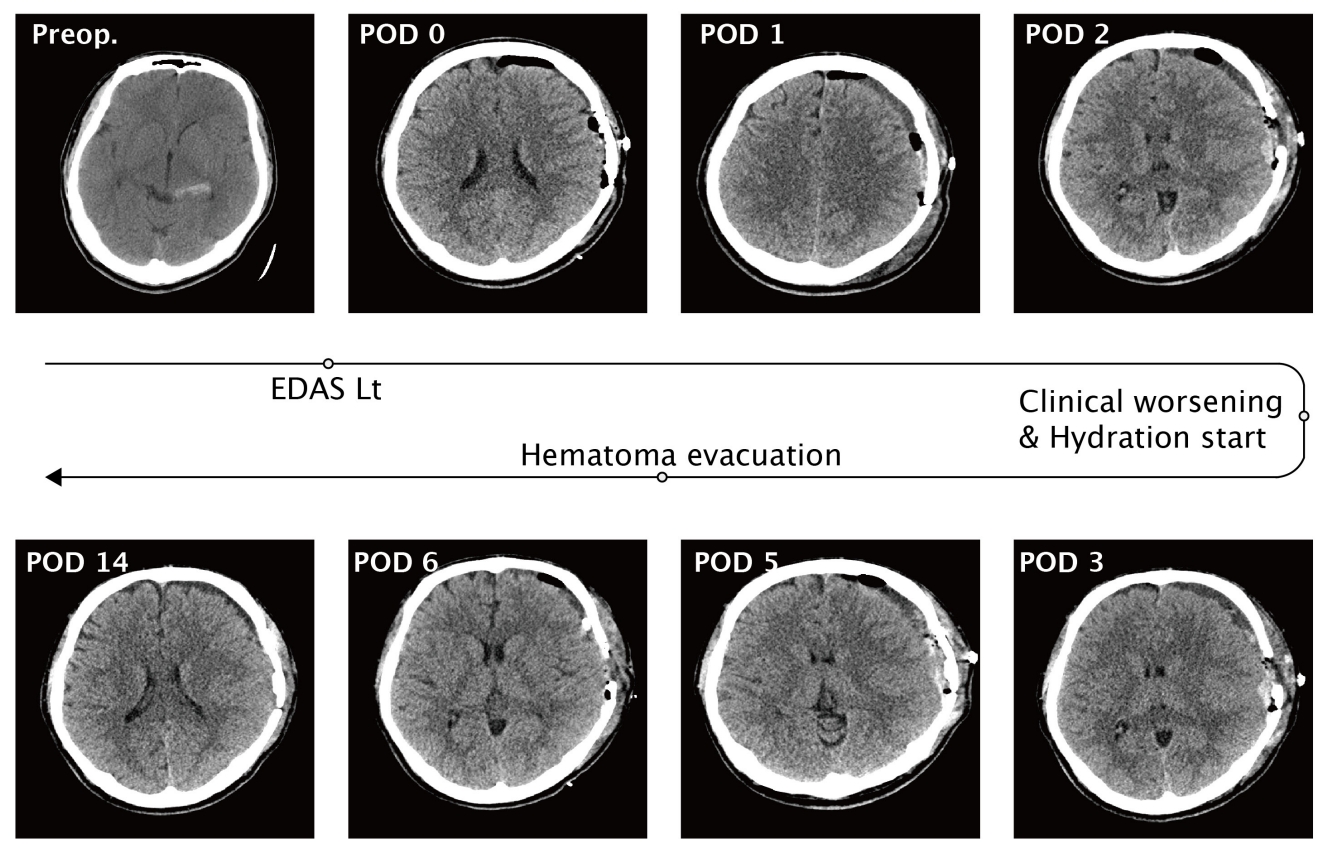
Fig. 2.
Chronological angiogram images - Preoperative; 2.42 mm sized saccular aneurysm was identified on his plexal segment of left anterior choroidal artery, Postoperative day 17, during massive hydration; Enlarged aneurysm of maximal diameter 5.33 mm, Postoperative day 35, after external carotid collateral formations; Dramatical size decrement of aneurysm.

REFERENCES
1. Borota L, Marinkovic S, Bajic R, Kovacevic M. Intracranial aneurysms associated with Moyamoya disease. Neurol Med Chir (Tokyo). 1996 Dec;36(12):860-4.


2. Coughlin DJ, Miller CA, Schuette AJ. Treatment of Moyamoya disease and unruptured intracranial aneurysm in floatingharbor syndrome. World Neurosurg. 2017 Aug;104:1049.

3. Teixeira BCA, Cardoso-Demartini AA. Choreoathetosis in Moyamoya disease. World Neurosurg. 2021 Dec;156:103-4.


4. Duan L, Bao XY, Yang WZ, Shi WC, Li DS, Zhang ZS, et al. Moyamoya disease in China: its clinical features and outcomes. Stroke. 2012 Jan;43(1):56-60.


5. Kim JH, Kwon TH, Kim JH, Chong K, Yoon W. Intracranial aneurysms in adult Moyamoya disease. World Neurosurg. 2018 Jan;109:e175-82.


6. Kim S, Jang CK, Park EK, Shim KW, Kim DS, Chung J, et al. Clinical features and outcomes of intracranial aneurysm associated with Moyamoya disease. J Clin Neurol. 2020 Oct;16(4):624-32.




7. Lee CY. Embolization with NBCA for ruptured aneurysm located in the Moyamoya-like collateral network associated with isolated middle cerebral artery occlusion. Asian J Neurosurg. 2018 Oct-Dec;13(4):1236-8.



8. Matsushima Y, Qian L, Aoyagi M. Clin Neurol Neurosurg. Asian J Neurosurg. 1997 Oct;99 Suppl 2:S19-22.
9. Takahashi JC, Miyamoto S. Moyamoya disease: recent progress and outlook. Neurol Med Chir (Tokyo). 2010 50(9):824-32.


10. Yamada H, Saga I, Kojima A, Horiguchi T. Short-term spontaneous resolution of ruptured peripheral aneurysm in Moyamoya disease. World Neurosurg. 2019 Jun;126:247-51.


-
METRICS

-
- 4 Crossref
- 0 Scopus
- 1,736 View
- 50 Download
- ORCID iDs
-
Jang Hun Kim

https://orcid.org/0000-0001-8557-1627 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



