 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(3); 2023 > Article |
|
Abstract
We describe a rare case of sacral epidural arteriovenous fistulas (edAVFs) with atypical clinical course of treatment. A 78-year-old man with a history of spinal surgery presented progressive gait disturbance and urinary incontinence. Spinal angiography demonstrated a sacral spinal AVF fed by bilateral lateral sacral arteries, draining to the venous pouch with subdural drainage. The first treatment by direct interruption of a subdural drainer was incompletely finished. Postoperative reassessment by 3D imaging analysis led to the diagnosis of sacral edAVF and 3D understanding of its angioarchitecture. The second treatment by transarterial embolization (TAE) resulted in complete occlusion of a sacral edAVF. However, spinal venous congestion didn’t improve, because the recruitment of occult edAVFs at the multiple lumbar levels and complex-shaped sacral ventral epidural venous plexus (VEP) were involved in the remnant of prior subdural drainage. The third treatment was performed by TAE for three occult edAVFs and the VEP compartment connecting between a patent edAVF and subdural drainage, which resulted in complete disappearance of spinal cord edema. Endovascular embolization of VEP compartment connecting to subdural drainage in addition to fistulous occlusion may be one of the treatment options for several edAVFs at the multiple spinal levels.
Spinal epidural arteriovenous fistulas (edAVFs) are commonly fed by multiple (multilevel and/or bilateral) feeders, and form a shunted venous pouch in the ventral epidural space at the single spinal level of lumbar vertebrae, draining into the subdural veins/ perimedullary veins or paravertebral veins [4,8,9,11,12,17]. Because their angiographic and clinical characteristics mimic dural AVFs (dAVFs), spinal edAVFs could often be misdiagnosed and treated as spinal dAVFs, which could be involved in the treatment failure [6,8,17]. Compared with other spinal levels, in particular, the difficulty in treatment of sacral edAVFs are often characterized by its rarity as spinal arteriovenous shunt disease, the similarity with sacral dAVFs [1,3,14,16], and the complexity of epidural venous drainage system due to the unique anatomical feature of sacral ventral epidural venous plexus (VEP) [5,9,10,15]. In the present case report, we describe a rare and difficult-to-treat case of a sacral edAVF with concomitant occult multiple lumbar edAVFs.
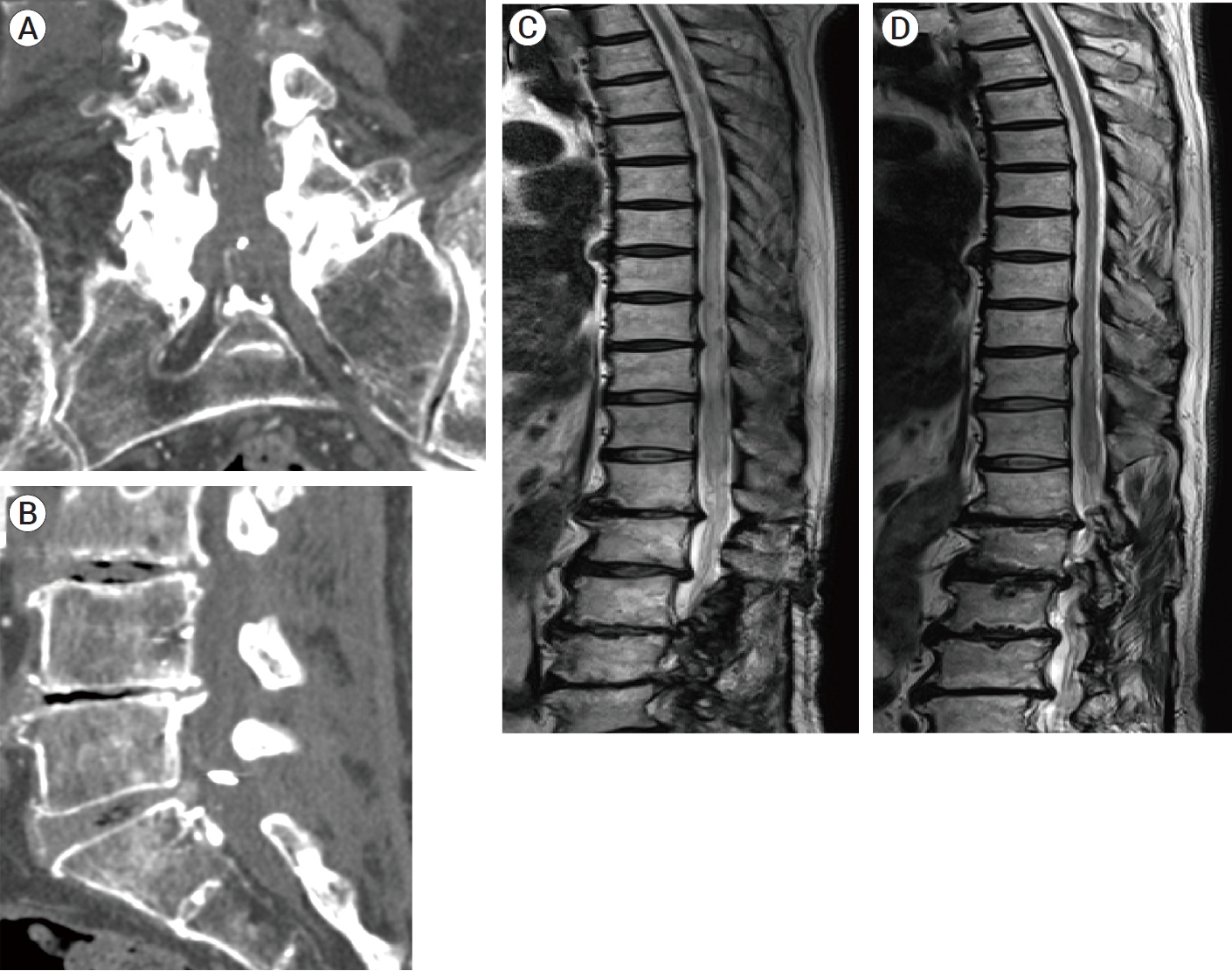
A 78-year-old man presented with a two-year history of progressive gait disturbance and urinary incontinence. He was diagnosed with lumbar spinal canal stenosis, and then underwent posterior lumbar decompression at the L3/4 spinal level. While the improvement of paraplegia was confirmed postoperatively six weeks after the operation, the deterioration of paraplegia relapsed (muscle strength 2/5). MRI showed T2 signal changes and marked enlargement of the spinal cord with dilated, tortuous veins below the Th 3/4 level (Fig. 1A). The patient was suspected of a spinal dAVF and was referred to our institution. Spinal angiography demonstrated that feeding branches from bilateral lateral sacral arteries collected to the venous pouch at the L5-S1 spinal levels (Fig. 1B, C and D). In addition, a subdural ascending vein arising from the venous pouch was detected. The involvement of median sacral arteries and other segmental arteries from Th12 to L5 was not confirmed. The patient was diagnosed with a sacral dAVF.
This patient underwent microsurgical clip closure of a subdural drainer. Intraoperatively, a spinal surgeon explored intradural space at the L5/S1 spinal level and detected a relatively thick radicular vein hidden by the nerve roots. The arterialization of the target radicular vein was identified by indocyanine green (ICG) videoangiography (Fig. 1E), and two Weck® ligating clips (Teleflex, Research Triangle Park, USA) were (Fig. 1F). Doppler flowmetry revealed the disappearance of arterial blood flow signal. Postoperatively, his symptoms and MRI findings did not show any interval change. After a week of the operation, spine CTA (Fig. 1G and H) and spinal catheter angiography (Fig. 1I) demonstrated that the clipped subdural drainer was still patent, and insufficient clip closure was suspected. Regarding the retreatment of the sacral arteriovenous shunt disease, the patient was consulted to an endovascular team.
Postoperative reassessment of sacral arteriovenous shunt disease was performed using multi-detector CTA imaging. Unenhanced and enhanced CT data were transferred to a workstation, and the image analysis software (Ziostation2, Ziosoft Inc, Japan) was used to generate digital subtraction CT angiographic data. All CT data sets were reformatted by using maximum intensity projection (MIP) and 3D multifusion imaging technique. Digital subtracted MIP images and 3D multifusion images revealed that a wide range of venous pouch was found to be the enlarged ventral epidural venous plexus (VEP) with a hexagonal shape (Fig. 2A, B, C and D). In addition, 3D multifusion imaging demonstrated that a subdural drainer arose from the lower compartment of a hexagonal sacral VEP (Fig. 2E and F). We diagnosed this as a sacral edAVF with both perimedullary drainage and paravertebral drainage. According to the prior catheter angiography (Fig. 1B, C and D), feeders collected to the upper compartment of sacral VEP. Therefore, the origin of a subdural drainer was found to be very distant from the VEP compartment with shunt points. We thought that the complete fistulous occlusion by transarterial embolization (TAE) could lead to the treatment success for the remaining sacral edAVF without the occlusion of a subdural drainer.
Under local anesthesia, a 4-Fr 11-cm introducer sheath was inserted into the right femoral artery. A 4-Fr Cobra type (C2) catheter with kink resistance (Nishiya; Medikit, Tokyo, Japan) was placed into the left internal iliac artery. Angiography of a left internal iliac artery showed two feeding branches from a left lateral sacral artery and a shunted VEP with subdural drainage. Angiography of the contralateral internal iliac artery also showed feeding branches from a right lateral sacral artery collected to the shunt point on the same VEP compartment. A 1.5-Fr microcatheter (ASAHI Veloute Ultra®; ASAHI INTECC, Aichi, Japan) with a microguidewire (ASAHI CHIKAI 14®; ASAHI INTECC, Aichi, Japan) was navigated up to feeders branching from a left lateral sacral artery and placed as close to the fistula as possible. Superselective catheter angiography clearly showed that the shunt points were located on the upper compartment of the hexagonal sacral VEP and there were the two-lane venous connection between a lumbar and a sacral VEP (Fig. 3A and B). Subsequently, 11% warmed n-butyl-2-cyanoacrylate (NBCA) was injected from every feeder. At the first injection, the penetration to the upper compartment of sacral VEP through the fistula was achieved (Fig. 3C). At the second injection, some degree of NBCA was filling to the venous pouch, and subsequently injected NBCA glue was penetrated to the contralateral feeders in a retrograde fashion via the retrocorporeal anastomosis (Fig. 3D). As expected, NBCA glue did not reach a subdural drainer. Angiography of bilateral internal iliac arteries did not show any feeders and the shunted epidural venous drainage (Fig. 3E and F), indicating the complete occlusion of a sacral edAVF. Two weeks after the second treatment, spine CTA showed the visualization of patent sacral VEP with a previously observed subdural drainer was still remaining (Fig. 3G and H). MRI did not show any interval change of spinal cord edema. Diagnostic spinal angiography including superselective exploration revealed that another four edAVFs newly appeared on longitudinal VEP at the L4-L5 spinal levels (Fig. 4A, 5A, B, C and D). Feeding branches originating from right L3, bilateral L4, and L5 segmental arteries were involved in these edAVFs. Superselective angiography of L3, L4, and L5 segmental arteries showed that the shunt blood flow from these edAVFs drained into the longitudinal VEP with ladder-shape between L4 and S1 spinal levels. However, a previously observed subdural drainer could not be visualized by angiography of L3-L5 segmental arteries, probably because the origin of a subdural drainer was away from every occult fistula. Angiography of bilateral internal iliac arteries demonstrated that sacral edAVF treated by prior TAE stayed completely occluded. According to imaging data obtained so far, there were two-lane venous drainage routes in the upper compartment of sacral VEP (Fig. 2D, 3B and 4A). Of the two drainage routes, one lane was already occluded with NBCA casts while the other lane remained patent, indicating that epidural venous drainage connection between new edAVFs and the prior subdural drainer remained (Fig. 4A). Therefore, shunt blood flow from newly emerged lumbar edAVFs could reach a subdural drainer through the remaining patent drainage rout of sacral VEP, which might result in the remnant of spinal venous congestion.
Following diagnostic angiography, we retried TAE in order to embolize new fistulas and the venous drainage route of VEP towards a subdural drainer within the possible range. Although direct NBCA penetration to a subdural drainer is actually impossible, we expected that the interruption of epidural venous drainage connection between new fistulas and a subdural drainer might result in resolving the spinal venous congestion. During the third treatment, a microcatheter was navigated to a feeder branching from a left L4 segmental artery and placed in the distal portion as close to the fistula as possible. Superselective angiography showed that feeders from a left L4 segmental artery collected to the F2 fistula and it also collected to the F3 fistula together with a contralateral feeder via the retrocorporeal anastomosis (Fig. 4A, 5C and D). The shunt blood flow from these fistulas ran downward through the longitudinal VEP toward the sacral spinal level (Fig. 5D). After the injection of 11% warming NBCA, NBCA glue penetrated into the VEP through these fistulas. A wide range of VEP at the L4 spinal level was filled thickly with NBCA casts (Fig. 5E). Subsequently, a microcatheter was guided to a feeder branching from a right L3 segmental artery to embolize the F1 fistula. Injection of 11% warming NBCA resulted in the feeder occlusion. Finally, a microcatheter was guided to a feeder branching from a left L5 segmental artery to embolize F4 fistula. A microcatheter was successfully placed in the distal portion as close to the fistula as possible. After the injection of 11% warming NBCA, NBCA glue slightly penetrated into the VEP through the F4 fistula and additionally penetrated to contralateral feeders via the retrocorporeal anastomosis. The F4 fistula was completely occluded because NBCA penetration to VEP through the F4 fistula was slightly achieved. We eventually embolized three newly emerged fistulas (F2, F3, and F4) and a prior F0 fistula (Fig. 4B). Although the occlusion of F1 fistula failed, the direct epidural venous drainage connection between remaining F1 fistula and a subdural drainer was blocked by NBCA casts filled with a part of lumbar VEP compartment (Fig. 4B and 5F). After a week of the third treatment, spine CTA showed the disappearance of an arterialized subdural drainer (Fig. 6A, B). Follow-up MRI after 3 months (Fig. 6C) and 10 months (Fig. 6D) from the third treatment showed that spinal cord edema gradually decreased and eventually disappeared without any vessel flow voids.
Spinal edAVFs were poorly understood and tended to be misdiagnosed as spinal dAVFs [6,8,17]. Especially at the sacral spinal level, the discrimination between dAVFs and edAVFs may be more difficult, because the angiographic feature of sacral dAVFs was reported to show more similarity with edAVFs, compared with typical thoracolumbar dAVFs [14,16]. Differently from other spinal levels, sacral dAVFs can often have multiple feeders, both ipsilaterally and bilaterally [13,14,16]. In addition, as for the venous drainage system, although the presence of shunted venous pouches was reported to be the specific feature of spinal edAVFs differently from spinal dAVFs [8], several literature reported that shunted venous pouches with perimedullary venous drainage could be also seen in sacral dAVFs [1,13,16]. Therefore, regarding especially sacral spinal arteriovenous lesions, the detailed evaluation of 3D anatomical relationship among feeders, shunt points, and venous drainage system using CTA and 3D rotational angiography was reported to be important for the accurate diagnosis [7,8]. In the present case, at the first treatment, the patient was directly operated as a spinal dAVF, based on the results of MRI and 2D-digital subtraction angiography (DSA). A spinal surgeon had made a surgical plan based on only 2D angiograms without the evaluation of 3D angioarchitecture, because his main concern was the location of a target subdural drainer. As the anatomical feature of spinal dAVF, the dural penetration site of a draining vein was found to be usually within 1 cm from the shunt point [10,11]. Therefore, a spinal surgeon might have thought that he had only to perform the direct approach to L5/S1 spinal level and he could easily find a draining vein piercing the dura. However, it was reported that intraoperative impressions for a target subdural drainer were somewhat different whether spinal arteriovenous lesion was dAVF or edAVF [2,3]. Burkhardt et al. [2] reported that spinal dAVF is located in the dura at the root sleeve and it drains directly from the shunt point on dura mater into markedly dilated single vein, while the draining vein from spinal edAVF is smaller and less prominent than that from spinal dAVF, probably because of remoteness from the fistula and multiple venous outflow channels. They warned that the draining vein of spinal edAVF was intermingled with the nerve roots, and a target arterialized vein was more difficult to identify intraoperatively [2]. In our case, we could not detect a draining vein penetrating dorsal dura mater, and additionally several radicular veins intermingled with the nerve roots was confirmed. An operator managed to find an arterialized vein using ICG video angiography, and he just performed the clip closure without coagulating and dividing a radicular vein, because he felt strange about the preoperative diagnosis of spinal dAVF. The failure of the first treatment was deemed due to the under-recognition of spinal edAVFs.
During the second treatment, we tried again to investigate the sacral arteriovenous lesion by using multimodal imaging technique and imaging analysis software, and we diagnosed this case as sacral edAVF with both perimedullary and paravertebral drainage. We reassessed the results of 3D CT angiography reconstructed with the image analysis software Ziostation2 in addition to the results of catheter angiography, and we understood the 3D anatomical relationship among shunt points, whole shape of shunted VEP, and a subdural drainer. NBCA penetration to a subdural drainer by TAE was thought to be impossible. However, because the shunt points of sacral edAVF was single vertebral level and localized in one compartment of sacral VEP, complete obliteration of sacral edAVF by TAE was considered to result in resolving spinal venous congestion without the NBCA penetration to a subdural drainer. Following the complete occlusion of sacral edAVF, an arterialized subdural drainer was expected to become normalized or occluded. A Japanese clinical study of 81 patients with edAVFs reported by Takai et al. [17] demonstrated that the glue penetration to an epidural venous pouch through the fistula was sufficient for the treatment of spinal edAVF, regardless of whether the injected glue reached and occluded the subdural drainer. In addition, they also reported that the fistulous connection between feeder(s) and the dilated epidural venous plexus was located at a single spinal level in all 81 cases [17]. In the present case, at the second treatment we could penetrate NBCA into the epidural venous pouch and obliterate the sacral edAVF at the single spinal level, which seemed to achieve treatment success, according to the report from the prior Japanese clinical study. However, occult four edAVFs fed by L3-L5 segmental arteries newly appeared unexpectedly. Additionally, the shunt blood flow ran through a wide range of longitudinal VEP from L4 to S1 spinal levels. VEP of the sacral spinal level showed a large hexagonal shape with a two-lane route partially, and therefore there remained a venous drainage connection toward a subdural drainer via non-occlusive sacral VEP. The recruitment of occult edAVFs and remaining venous drainage route toward a subdural drainer were the cause of second treatment failure.
During the third treatment, we could occlude three edAVFs, and additionally occlude a part of lumbar VEP by TAE in order to block the epidural venous connection between non-occlusive edAVF and a subdural drainer. Previous literature reported that sacral VEP consisted of multiple venous drainage routes with plexiform structure, different from other spinal levels [5,9,10,15]. Even in our case, sacral VEP showed a complex structure such as a large hexagonal shape with a two-lane route. The complex shape of sacral VEP can have multiple venous drainage routes toward subdural drainage. Therefore, the well-planned embolization of VEP compartment may be needed if it is difficult to occlude a subdural drainer. The anatomical feature of sacral VEP can be one of important factors for planning the treatment strategy of sacral edAVFs. In our case, by the interruption of epidural venous connection between newly appeared edAVFs and a subdural drainer, we eventually obtained the almost complete disappearance of spinal cord edema and vascular flow voids in the follow-up MRI. However, the limitation of this case report is its short follow-up period. Further follow-up is certainly necessary for evaluating the validity of our endovascular treatment.
First, the 3D understanding of whole pathologic angioarchitecture is needed for the treatment of spinal edAVFs. The integrated analyses by using imaging data obtained from CTA in addition to MRI and catheter angiography should be performed. In particular, digital subtracted MIP images produced from CTA is useful to make the visualization of a remnant subdural drainer which can not be visualized even by catheter angiography. Second, the assessment of venous drainage routes including complex-shaped sacral VEP is important for treating sacral edAVFs. Finally, if the spinal venous congestion remains unresolved after embolizing the target spinal edAVF, the recruitment of occult edAVFs at the other spinal levels and the presence of remaining epidural venous drainage routes toward subdural drainage should be excluded.
Fig. 1.
(A) T2-weighted sagittal image of spine MRI, showing spinal cord swelling and vascular flow voids. (B-D) Preoperative angiography (anterior-posterior view) of right and left internal iliac arteries (B: subtracted digital image, C: non-subtracted digital image, D: subtracted digital image). Bilateral lateral sacral arteries collected to the shunt points on the upper compartment of hexagonal- shaped venous pouch and a subdural drainer (white arrow) arising from the venous pouch ran upward. (E-F) Intraoperative photograph during intradural exploration. (E) A radicular vein (white arrow) intermingled with the nerve roots was suspicious of a target subdural drainer. Indocyanine green videoangiograpy (presented in the small boxed area) demonstrated that the radicular vein was arterialized. (F) The arterialized radicular vein was clipped by two Weck® ligating clips (Teleflex, Research Triangle Park, USA). (G-I) Postoperative digital-subtraction CTA images and digital-subtraction angiogram. (G) Coronal MIP image and (H) sagittal MIP image produced from digital-subtraction CTA data, indicating the remnant of a subdural drainer (white arrow) despite the closure by two clips (white arrowhead). (I) Postoperative digital-subtraction angiogram (anterior-posterior view) of a right internal iliac artery showed that a residual subdural drainer (white arrow), while its visualization seemed to reduce due to the clip interruption. L5, fifth lumbar vertebrae; Sa, sacral vertebrae; MIP, maximum intensity projection
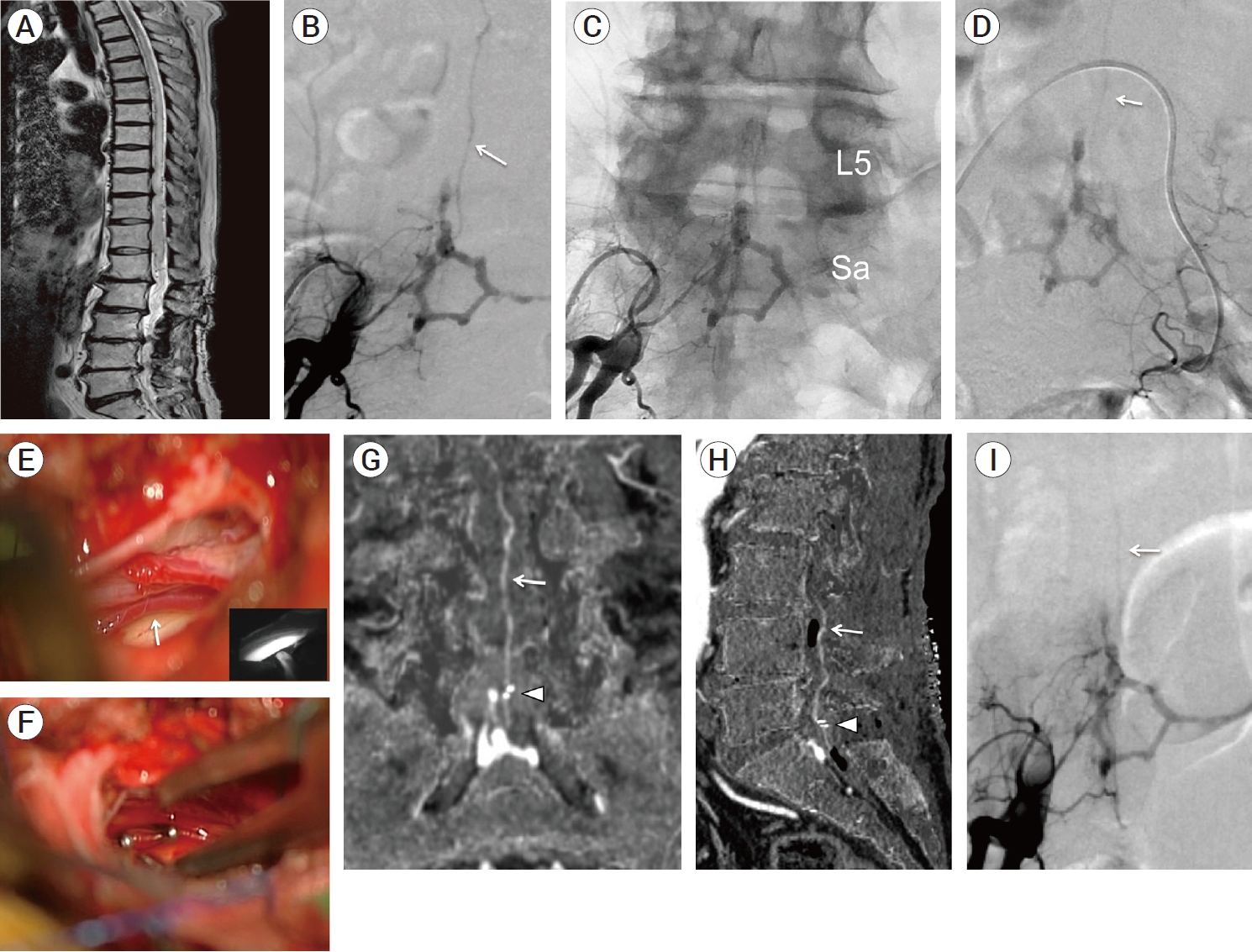
Fig. 2.
(A-C) Postoperative MIP images produced from digital-subtraction CTA data (A: coronal section, B: sagittal section, C: axial section), showing that a hexagonal venous pouch with subdural drainage was found to be the enlarged VEP at the L5-S1 spinal levels. (D-F) Postoperative 3D multifusion images produced from CTA data (D: anterior-posterior view, E: magnified left posterolateral view, F: magnified posterior-anterior view), showing the positional relationship among VEP (orange color), a subdural drainer (red color), Weck® ligating clips (Teleflex, Research Triangle Park, USA) (green color) and a lumbosacral vertebrae. 3D multifusion images also revealed that a subdural drainer arose from the lower compartment (black arrow) of sacral VEP, indicating that the origin of a subdural drainer was very distant from the shunt points (black arrowhead) on the upper compartment of sacral VEP. MIP, maximum intensity projection; VEP, ventral epidural venous plexus; L5, fifth lumbar vertebrae
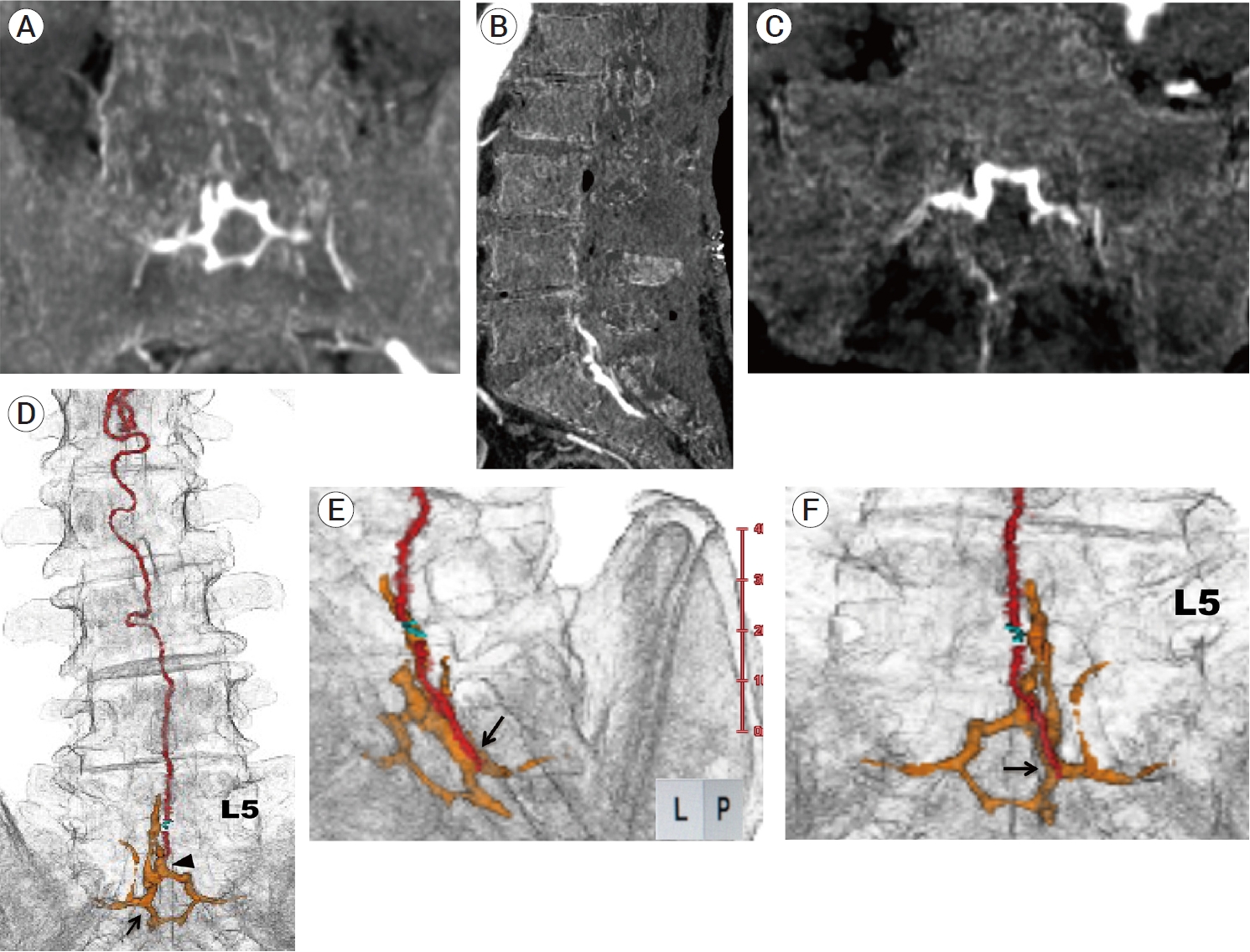
Fig. 3.
(A-C) Superselective angiograms of a feeder branching from a left lateral sacral artery during the first session of endovascular treatment. (A) In the arterial early phase, a hexagonal-shaped VEP and a shunt point (black arrowhead) were clearly observed. (B) In the arterial late phase, a two-lane route (asterisk) of venous drainage in the upper part of a hexagonal-shaped sacral VEP appeared in a slightly delayed fashion, indicating that there was a two-lane venous connection between a lumbar and a sacral VEP. (C) The first injection of 11% warming NBCA from a feeder branching from a left lateral sacral artery resulted in the glue penetration to the upper compartment of sacral VEP. White arrowhead indicates the tip of microcatheter. (D) The second injection of 11% warming NBCA from another feeder branching from a left lateral sacral artery resulted in a slight glue penetration through another shunt point to the remaining upper compartment of sacral VEP, followed by the glue penetration to contralateral feeders (black arrow) via the retrocorporeal anastomosis. (E, F) Angiograms of bilateral internal iliac arteries (E: right side, F: left side) showed the complete occlusion of the sacral edAVF. (G, H) MIP images produced from digital-subtraction CTA data (G: coronal section, H: sagittal section) after the first session of endovascular treatment, showing that a subdural drainer became poorly visualized but still remaining (white arrow). VEP, ventral epidural venous plexus; NBCA, n-butyl-2-cyanoacrylate; MIP, Maximum intensity projection; edAVF, epidural arteriovenous fistula
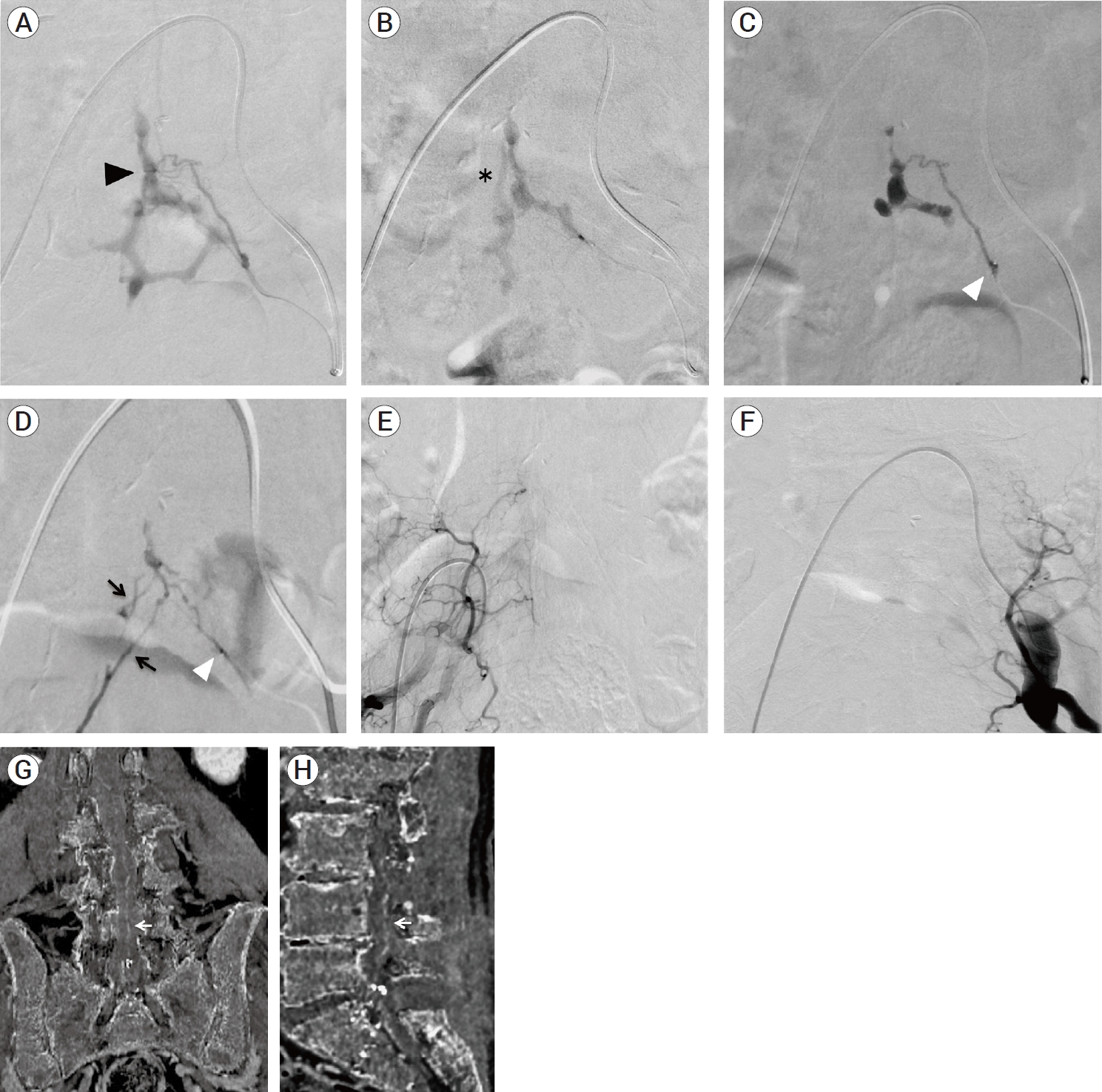
Fig. 4.
Schematic illustration of whole vascular architecture related to multiple spinal edAVF. (red color line: feeders, blue color band line: longitudinal VEP, yellow color line: a subdural drainer, black arrowhead: Weck® ligating clips (Teleflex, Research Triangle Park, USA), orange cross mark: fistula point). Every fistula is numbered from 0-4 in the cream color. Asterisk indicates a two-lane venous route between lumbar and sacral VEP. Black arrow indicates the origin of a subdural drainer. (A) After the first session of endovascular treatment. A part of sacral VEP, F4 fistula, and feeders filling with NBCA casts were represented by navy blue color. The recruitment of occult four fistulas (F1- F4) fed by L3-L5 segmental arteries was newly observed. Shunt blood flow from new four fistulas drained into the lumbar VEP with ladder- shape. Shunt blood flow from lumbar edAVFs ran downward to the sacral VEP. (B) After the second session of endovascular treatment. The compartment of longitudinal VEP, fistulas, and feeders filling with NBCA casts were represented by navy blue color. Eventually, four fistulas (F0 and F2-F4) were completely occluded by two sessions of endovascular treatment. Although F1 fistula was patent due to feeder occlusion, shunt blood flow from F1 fistula was blocked by NBCA casts. The epidural venous connection between all fistulas (F0-F4) and a subdural drainer was interrupted by TAE, suggesting that an arterialized subdural drainer became normalized or occluded theoretically. edAVF, epidural arteriovenous fistula; VEP, ventral epidural venous plexus; NBCA, n-butyl-2-cyanoacrylate; TAE, transarterial embolizaion; SA, segmental artery
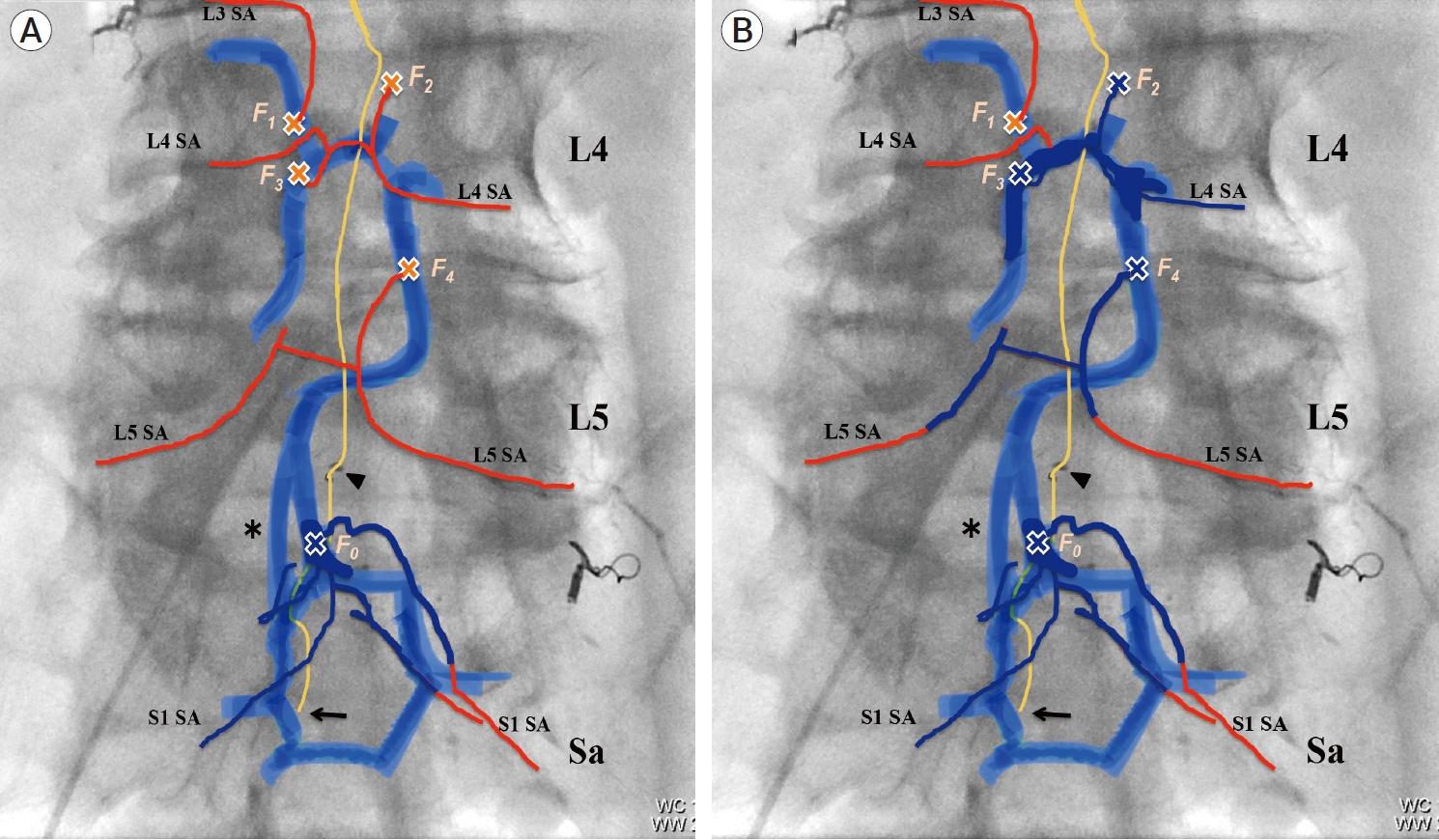
Fig. 5.
Representative angiograms from (A-E) a left L4 segmental artery and (F) a right L3 segmental artery. (A-B) Angiograms of a left L4 segmental angiography (A: before TAE, B: after the first session of TAE). After the first session of TAE, the shunted lumbar VEP (white double arrows) newly appeared. (C-D) Superselective angiograms from the distal portion of a left L4 segmental artery (C: arterial early phase, D: arterial late phase). The asterisk indicates the tip of microcatheter. The white single arrows indicate feeders branching from a left L4 segmental artery. The white triple arrows indicate a feeder branching from a right L4 segmental artery. These angiograms showed the early filling of ladder shaped VEP via F2 (white double arrowheads) and F3 fistulas (white single arrowhead), followed by descending epidural venous drainage (white double arrows) toward sacral VEP. (E) Fluoroscopic image after TAE, showing NBCA casts (white open arrowheads) filling in the partial VEP including F2 and F3 fistulas. (F) Angiogram of right L3 segmental angiography, showing the prior NBCA casts (white dotted ellipse) blocked the shunt blood flow from F1 fistula (black single arrow) toward ladder shaped VEP. The shunt blood flow from F1 fistula connected to only the paravertebral drainage (black double arrow). Black arrowhead indicates a feeder branching from right L3 segmental artery. TAE, transarterial embolization; VEP, ventral epidural venous plexus; NBCA, n-butyl-2-cyanoacrylate
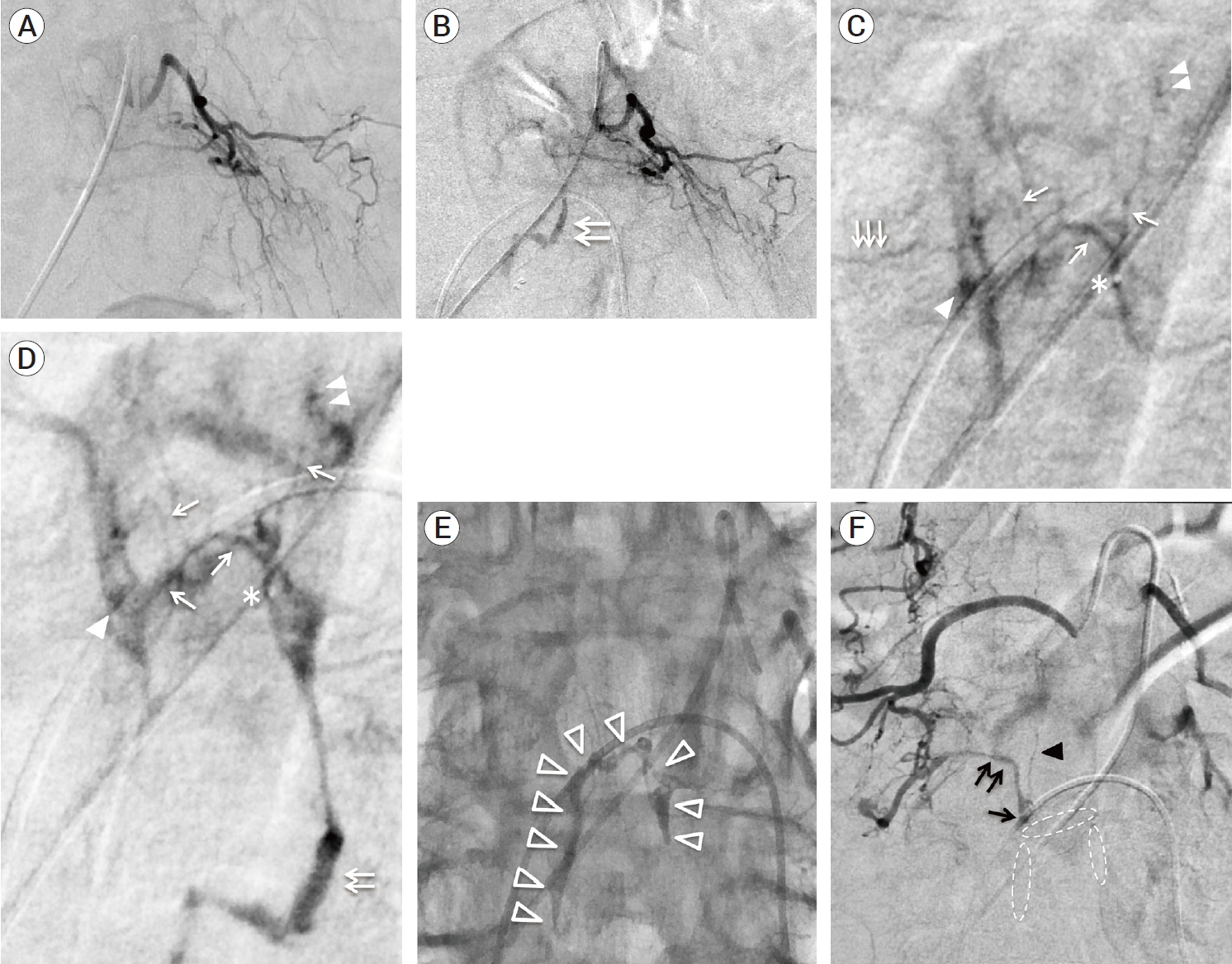
Fig. 6.
Postoperative follow-up CTA and MRI after the third treatment. (A, B)
MIP images produced from CTA data (A: coronal section, B: sagittal section),
showing the disappearance of a subdural drainer. (C, D) T2-weighted sagittal
images of spine MRI after the third treatment (C: after three months, D: after
ten months), showing that spinal cord edema gradually decreased and eventually
disappeared without any vessel flow voids. MIP, maximum intensity
projection
REFERENCES
1. Alvarado AM, Haussen DC, Ebersole K, Nogueira RG, Abraham MG. Embolization of sacral dural arteriovenous fistulas: a case series and literature review. Interv Neurol. 2017 Mar;6(1-2):73-81.




2. Burkhardt J, Safaee MM, Clark AJ, Lawton MT. Sacral epidural arteriovenous fistulas: imitators of spinal dural arteriovenous fistulas with different pathologic anatomy: report of three cases and review of the literature. Acta Neurochir. 2017 Jun;159(6):1087-92.



3. Clarke MJ, Patrick TA, White JB, Cloft HJ, Krauss WE, Lindell EP, et al. Spinal extradural arteriovenous malformations with parenchymal drainage: venous drainage variability and implications in clinical manifestations. Neurosurg Focus. 2009 Jan;26(1):e5.

4. Geibprasert S, Pereira V, Krings T, Jiarakongmun P, Toulgoat F, Pongpech S, et al. Dural arteriovenous shunts: a new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke. 2008 Oct;39(10):2783-94.


5. Groen RJ, Groenewegen HJ, van Alphen HA, Hoogland PV. Morphology of the human internal vertebral venous plexus: a cadaver study after intravenous araldite CY 221 injection. Anat Rec. 1997 Oct;249(2):285-94.


6. Hiramatsu M, Sugiu K, Yasuhara T, Hishikawa T, Nishihiro S, Kidani N, et al. Comparison between spinal dural arteriovenous fistula and spinal epidural arteriovenous fistula. Journal of Neuroendovascular Therapy. 2019 Mar;13(3):114-9.

7. Kiyosue H, Ide S, Uchida S, Kubo T. Dural arteriovenous fistulas: interpretation from the view point of vascular anatomy. No Kekkannai Chiryo. 2020 Jan;5(1):6-18.
8. Kiyosue H, Matsumaru Y, Niimi Y, Takai K, Ishigro T, Hiramatsu M, et al. Angiographic and clinical characteristics of thoracolumbar spinal epidural and dural arteriovenous fistulas. Stroke. 2017 Dec;48(12):3215-22.



9. Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009 Apr;30(4):639-48.



10. Lasjaunias P, Berenstein A, ter Brugge KG. Spinal and Spinal Cord Arteries and Veins. Clinical Vascular Anatomy and Variations. Volume 1, 2nd edition. Berlin, Heidelberg: Springer; 2001. p. 73-160.
11. Lenck S, Nicholson P, Tymianski R, Hilditch C, Nouet A, Patel K, et al. Spinal and paraspinal arteriovenous lesions. Stroke. 2019 Aug;50(8):2259-69.


12. Patsalides A, Knopman J, Santillan A, Tsiouris AJ, Riina H, Gobin YP. Endovascular treatment of spinal arteriovenous lesions: beyond the dural fistula. AJNR Am J Neuroradiol. 2011 May;32(5):798-808.



13. Ren Y, Liu H, Chen T, You C, Li J. Successful management of sacral dural arteriovenous fistulas: a case series and literature review. World Neurosurg. 2019 Jun;126:164-70.


14. Sasamori T, Hida K, Asano T, Aoyama T, Yamauchi T, Iwasaki M, et al. Sacral dural arteriovenous fistula. Spinal Surgery. 2011 Apr;25(1):81-3.

15. Tadie M, Hemet J, Aaron C, Bianco C, Creissard P, Huard P. Le dispositif protecteur anti-reflux des veines de la moelle. Neurochirurgie. 1979 25:28-30.
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,604 View
- 35 Download
- ORCID iDs
-
Katsuya Saito

https://orcid.org/0000-0002-5102-3080 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



