Endovascular recanalization therapy for patients with acute ischemic stroke with hidden aortic dissection: A case series
Article information
Abstract
Aortic dissection is one of the causes of acute ischemic stroke. Endovascular recanalization therapy (EVT) has emerged as an essential treatment for acute ischemic stroke due to large artery occlusion. However, it is rarely performed in the situation of hidden aortic dissection (AD). Two patients presented to the emergency room with focal neurologic deficits. The first patient was diagnosed with right internal carotid artery (ICA) occlusion. Angiography revealed that the ICA was occluded by the dissection flap. After a stent deployment in the proximal ICA, the antegrade flow was restored. The patient was diagnosed with AD on chest computed tomography (CT) after EVT. For the second patient, intraarterial thrombectomy was performed to treat left middle cerebral artery occlusion. AD was first detected on echocardiography, which was performed after EVT. Herein, we report successful endovascular recanalization therapy performed in two patients with acute ischemic stroke in the situation of undiagnosed aortic dissection. We also reviewed previous case reports and relevant literature.
INTRODUCTION
Aortic dissection (AD) is a catastrophic disorder with a mortality rate of up to 50% within 24 hours due to delayed or missed diagnoses [3,8]. Although the main symptom is the abrupt onset of typical chest pain, over 10% of patients do not experience this pain [2]. Stroke is a major complication of AD, and its diagnosis is often delayed in cases of stroke that abruptly present with neurological deficits.
Stroke is the initial manifestation of AD, particularly when the flap occurs in the ascending aorta and/or aortic arch [7]. The proposed mechanisms are direct extension into the carotid system, thromboembolism, and hemodynamic insufficiency. Endovascular recanalization therapy (EVT) is an essential treatment widely performed for acute ischemic stroke. However, it is rarely reported in cases of ischemic stroke with AD treated by EVT [4]. Herein we report two patients with acute ischemic stroke with extensive AD who underwent successful EVT.
CASE DESCRIPTION
Case 1
Initial presentation: An 81-year-old woman presented to the emergency room (ER) with left-sided weakness that started two hours prior. On neurologic examination, the patient was alert. Lower facial palsy, upper limb paralysis, and lower limb weakness of Medical Research Council (MRC) scale grade III were observed on the left side. The National Institutes of Health Stroke Scale (NIHSS) score was 9. She complained of chest pain, but the electrocardiogram (ECG) and cardiac enzyme test results did not indicate any significant abnormalities. Thus, brain imaging was performed immediately. Diffusion-weighted imaging revealed multifocal high signal intensity in the right parietal and frontal lobes (Fig. 1A), while perfusion imaging indicated significantly delayed perfusion in the entire right middle cerebral artery (MCA) region (Fig. 1B). Time-of-flight (TOF) angiography and T2-weighted images showed no visualization of right internal carotid artery (ICA) flow (Fig. 1C, D). There was a significant diffusion-perfusion mismatch, and the patient underwent endovascular treatment for large artery occlusion.
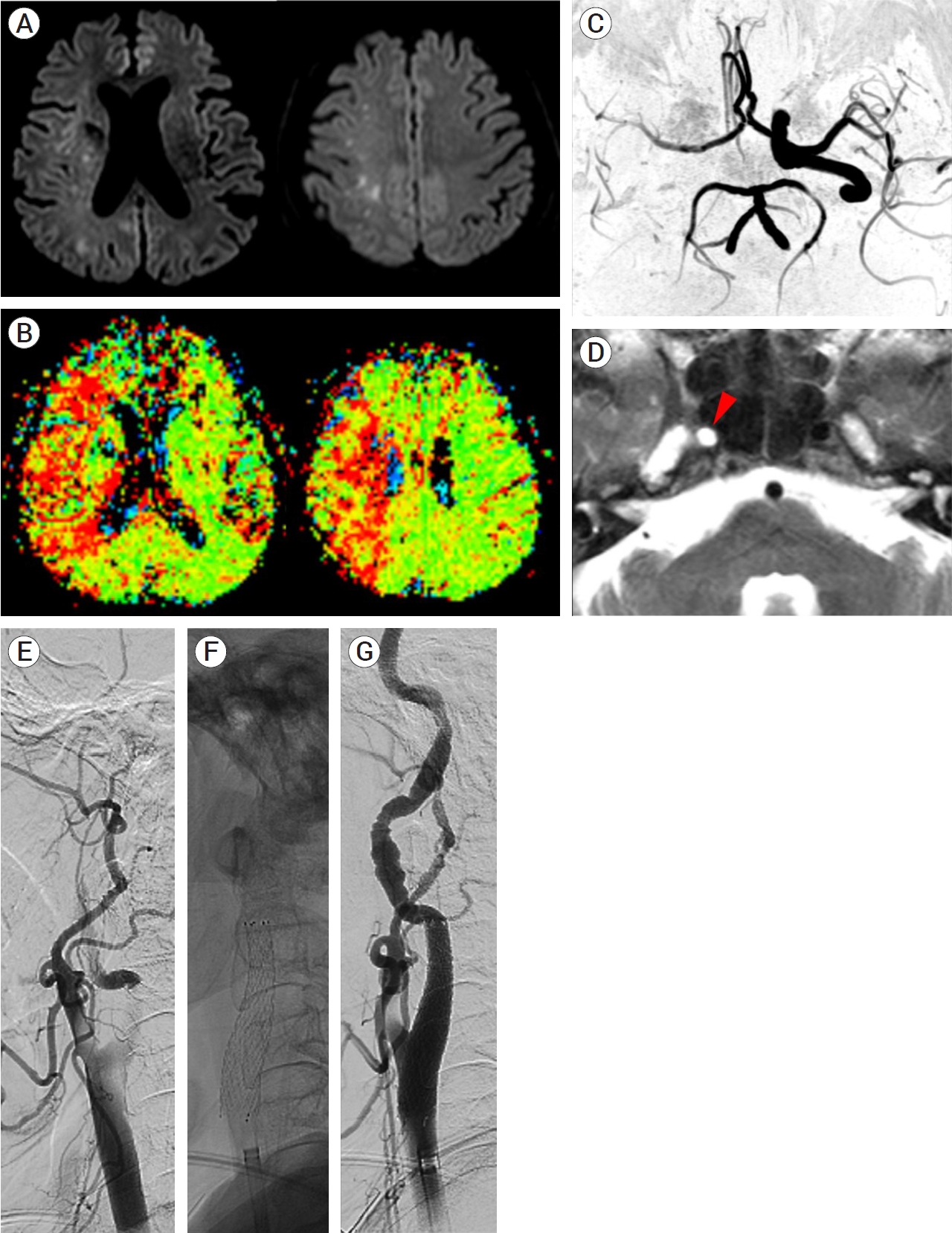
(A) Brain diffusion-weighted image showing multifocal high signal intensities in the right hemisphere. (B) Perfusion imaging revealing delayed mean transit time in the entire right MCA region. (C) Blood flow of the right ICA was not visualized on MR angiography. (D) The flow void within the right ICA was invisible during T2-weighted imaging (red arrowhead). (E) Digital subtraction angiogram showing the tapered flow from the distal CCA. (F) The stent was inserted and the CCA and ICA flow was restored (G). MCA, middle cerebral artery; ICA, internal carotid artery; CCA, common carotid artery
Endovascular procedure: During angiocatheter insertion into the ascending aorta from the femoral artery and manipulation for great vessel selection, the surgeon felt their action to be somewhat restricted compared to usual cases due to the narrow lumen of the aorta. Insufficient collateral flow to the right anterior cerebral artery (ACA) and MCA via the anterior communicating artery was noted during left ICA angiography. Right common carotid artery (CCA) angiography revealed complete occlusion of the ICA by the dissection flap at the proximal portion (Fig. 1E). The resulting false lumen was not significant until the proximal two-thirds portion of the CCA. One Protégé stent (Medtronic, MN, USA) 8×6×40 mm was deployed from the proximal ICA to the distal portion of the CCA using a SPIDER Embolic Protection Device (Medtronic, MN, USA) (Fig. 1F). The antegrade flow of the ICA was restored without further proximal stent deployment (Fig. 1G), and the procedure was discontinued after confirming that no distal occlusion was observed.
Postprocedural course: The patient was alert despite the persistence of left hemiparesis after the procedure. She complained of persistent chest pain, though there was no significant interval change in the ECG after the procedure. Computed tomography (CT) of the aorta was performed, which revealed DeBakey type I AD that extended from the proximal ascending aorta to the left renal artery level. The dissection flap also involved the proximal innominate artery without flow compromise and extended distally with a gradual diameter increase to the stented distal CCA segment, also without flow compromise (Fig. 2A). The maximal diameter of the ascending aorta was approximately 5 cm, and the true lumen of the thoracoabdominal aorta was compressed eccentrically (Fig. 2B). The flap did not involve the left CCA or subclavian artery, which originated from the true lumen (Fig. 2C).
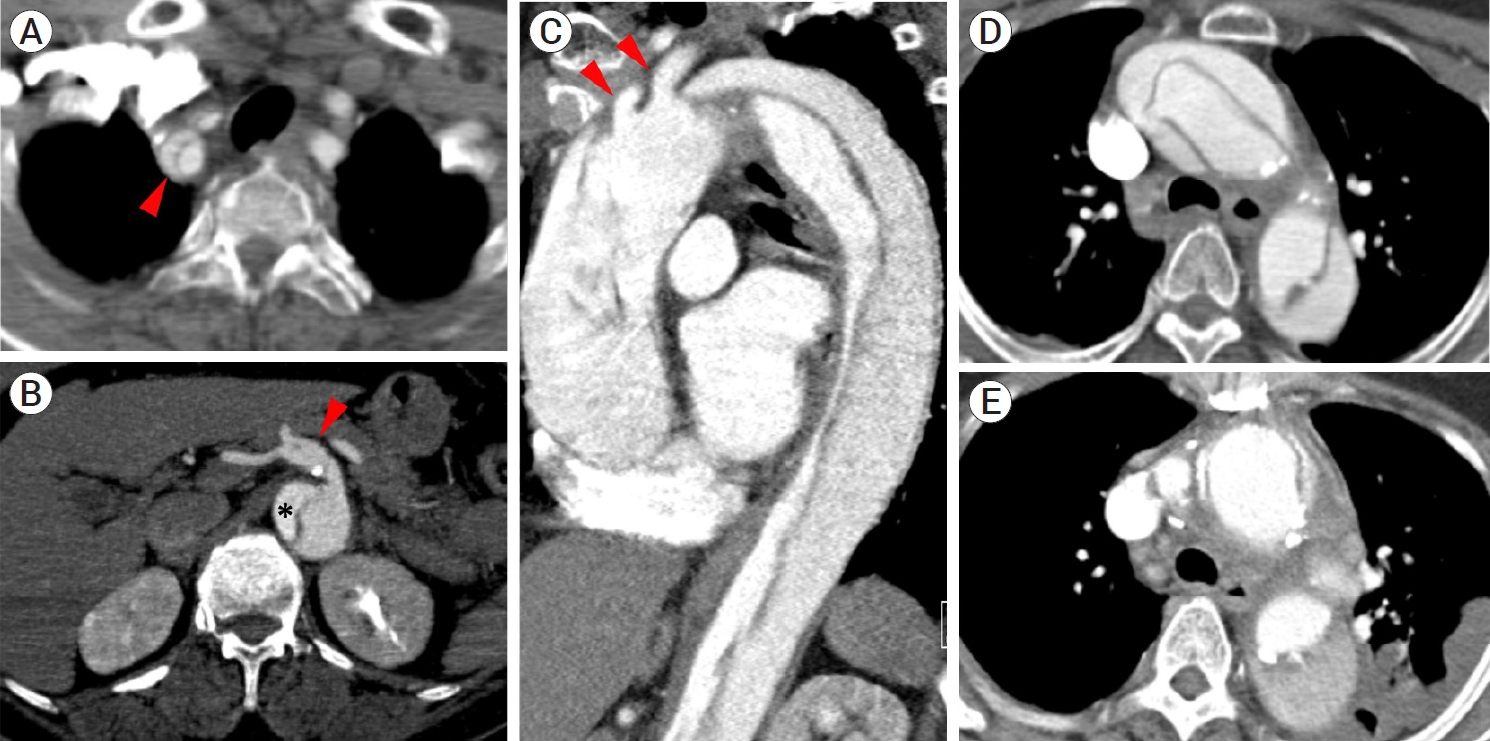
(A) Chest CT revealed aortic dissection originating in the ascending aorta. The flap involved the proximal innominate artery (red arrowhead). (B) The dissection propagated to the abdominal aorta (DeBakey type I) and superior mesenteric artery (red arrowhead) following its origin from the false lumen. The true lumen was compressed eccentrically along the thoraco-abdominal aorta (star marking). (C) The flap did not involve the left CCA or subclavian artery (red arrowheads), which originated from true lumen. (D) The dissection flap, which was observed in the ascending aorta and arch at diagnosis, was replaced after surgery (E). CT, Computed tomography; CCA, common carotid artery
The patient was transferred to another hospital that was available for emergent surgery. She underwent ascending aorta and partial arch replacement surgery and was transferred back to the department of rehabilitation medicine after three weeks of postoperative care. The follow-up CT revealed that the dissection flap, which was observed in the ascending aorta and arch at diagnosis, was replaced with the graft after the surgery (Fig. 2D, E). She was discharged one month later with mild weakness in her left arm (NIHSS score 1), which was significantly improved compared to her initial NIHSS score of 9.
Case 2
Initial presentation: A 76-year-old woman presenting with aphasia and right-sided weakness was found by a caregiver and presented to the ER. Neurologic examination found the patient to be alert but unable to cooperate due to global aphasia. The eyeballs deviated toward the left side, and lower facial paralysis was observed. She also could not move her limbs against gravity, so her overall NIHSS score was 16. She arrived at the ER five hours after the first known abnormal time, and brain perfusion imaging was performed immediately. Brain imaging revealed high signal intensity in the left basal ganglia and right parietal white matter (Fig. 3A), while perfusion imaging revealed reduced perfusion in the entire left MCA region (Fig. 3B). MR angiography showed occlusion of the proximal portion of the left MCA. Before the procedure, she had been prescribed warfarin due to the presence of a prosthetic aortic valve placed during AVR surgery six years prior, although her international normalized ratio (INR) was 1.5 in the initial blood test. The electrocardiogram (ECG) showed atrial fibrillation. Serum levels of cardiac enzymes were normal. There was a significant diffusion-perfusion mismatch on MRI, and the patient underwent endovascular treatment for large artery occlusion.
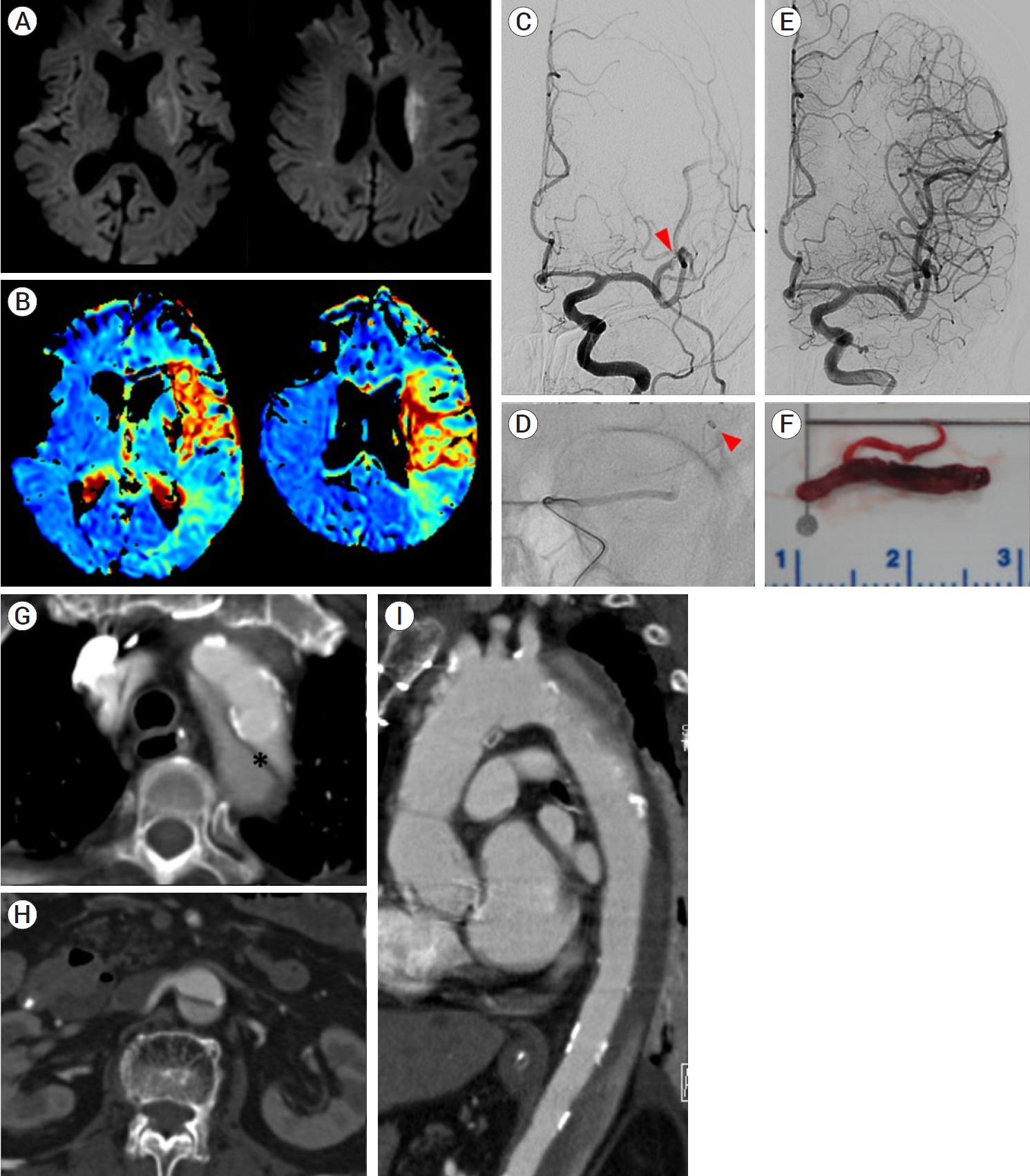
(A) Brain diffusion-weighted imaging showing high signal intensities in the left basal ganglia. (B) Perfusion image revealing delayed mean transit time in the superior branch region of the left MCA. (C) Digital subtraction angiogram showing occlusion in the superior branch of the left MCA (red arrowhead). (D) Intraarterial thrombectomy using a Catalyst 7 (Stryker, MI, USA) suction catheter was performed. Catheter tip is showing (red arrowhead). (E) After withdrawal of a huge thrombus, complete reperfusion was achieved (F). (G) Aorta CT showing the dissection flap (star marking) originating from the proximal descending aorta to both CIAs (DeBakey type III). (H) Important arteries to the internal organ originated from the true lumen of abdominal aorta. (I) The ascending aorta and aortic valve had already been surgically replaced. MCA, middle cerebral artery; CT, computed tomography
Endovascular procedure: The surgeon felt that the action of the angiocatheter tip was somewhat limited without significant hindrance during angiocatheter delivery to the aortic arch from the femoral artery. Digital subtraction angiography (DSA) revealed occlusion of the superior branch of the left MCA (Fig. 3C). Suction thrombectomy was performed using a Catalyst 7 (Stryker, MI, USA) suction catheter protected by a Flowgate [3] Balloon Guide Catheter. A large amount of thrombus was removed from the occluded site at the first pass, and the flow to the superior branch of the MCA was completely restored (Fig. 3D, E). No remaining occlusion of the distal branch was observed (Fig. 3F).
Postprocedural course: The patient’s neurologic deficits did not change immediately after the procedure. The following day, her aphasia improved slightly. She was partially cooperative without verbal output and right hemiparesis persisted. Echocardiography revealed a mobile linear flap between the descending thoracic and abdominal aorta. The prosthetic aortic valve functioned well. Aorta CT showed a dissecting flap from the proximal descending aorta to both common iliac arteries, which was classified as DeBakey type III AD (Fig. 3G), and fortunately, important arteries to the internal organs originated from the true lumen of the abdominal aorta (Fig. 3H). CT also revealed that the ascending aorta and aortic valve had already been replaced with mechanical prostheses (Fig. 3I). Upon consultation, the cardiac surgeon stated that no further surgical treatment was needed, and she continued to receive warfarin as anticoagulation therapy. The patient was transferred to the rehabilitation medicine department for comprehensive rehabilitation therapy for aphasia and right-sided hemiparesis.
DISCUSSION
Acute stroke physicians should always consider the possibility of AD, considering that the incidence of neurologic signs as the initial presentation of AD is reportedly 18–30%. However, diagnosing AD promptly is often challenging as the simultaneous occurrence of AD and acute ischemic stroke is extremely rare (0.3%). Of them, only 1.7% are hyperacute stroke patients that arrive at the hospital within four hours following symptom onset and can feasibly undergo thrombolytic therapy [4,10]. Hence, cases of EVT in the setting of AD are rarely reported [9]. Considering that AD can be fatal, physicians should be aware of the simultaneous occurrence of stroke and AD, especially when the patient cannot complain of any symptoms due to neurologic deficits.
In this case series, two patients presented to the ER with ischemic stroke within six hours following symptom onset. Both exhibited significant diffusion-perfusion mismatches with large artery occlusion and underwent successful EVT and carotid artery stenting, as well as suction thrombectomy. All procedures during EVT were performed smoothly, except that the surgeon felt limitations in the action of angiocatheter tip during advancement in the aorta. ADs were first diagnosed after emergent EVT was performed in both cases. The first patient complained of chest pain before EVT. However, there were no abnormalities in the initial cardiac evaluation, so AD was not suspected at first. The second patient did not complain of any symptoms due to aphasia, and there were no abnormalities in the initial cardiac evaluation. There was no interval change in the ECG or cardiac enzyme levels after the procedure in either case. As chest CT is not routinely performed in the ER for acute ischemic stroke patients, the diagnosis of AD was missed before EVT. However, these cases indicate that EVT with a femoral puncture is feasible in the situation of hidden AD.
Although EVT is an emerging safe and effective treatment modality for acute ischemic stroke with large artery occlusion, performing the procedure on patients with AD prior to corrective surgery is challenging. In addition to the increased risk of bleeding due to the systemic infusion of heparin during EVT, some technical considerations exist. First, the transfemoral approach is relatively safe because AD rarely involves at levels lower than the common iliac artery. However, determining the true lumen of the aorta can be challenging, especially when communication between the true and false lumens exists, as in our second case. The surgeon can feel when the actions of guiding-catheter or angio-catheter tips are limited during manipulation due to the narrowing of the aorta and arch. This feeling, even though it is an important clue that indicates AD, can easily be overlooked during the effort to rapidly proceed with recanalization therapy. When restricted catheter action is felt, catheter manipulation should be performed more carefully considering the possibility of further damage to the dissection flap during EVT. Other access routes, such as transbrachial and direct carotid puncture, should also be considered when the restriction may prevent the procedure from being performed successfully [5,6]. Second, the optimal stent deployment range should be considered carefully after confirming proximal flow compromise due to the dissection flap. In one case reported by Funakoshi et al., six stents were deployed along a long-dissected lesion as it ran from the innominate artery to the ICA. In another case involving CCA origin, three stents were deployed from the ICA to the origin of CCA to cover the entry tear [1]. In our first case, the dissection flap from the innominate artery origin to mid-CCA was, fortunately, not significant. Therefore, the compromised right ICA flow was restored by the deployment of only one stent from the distal CCA to the proximal ICA. Minimizing metallic stent implantation in the proximal great vessels as much as possible is helpful in the context of recanalization therapy and aortic arch surgery. Third, possible stent-retriever tangling with the stent should be considered when tandem occlusion of the distal arteries is noted after proximal carotid artery stenting and successful flow restoration. Delivery of the balloon guiding the catheter tip distal to the carotid stent is required to prevent tangling, and suction thrombectomy is a good alternative, as in our second case.
CONCLUSIONS
EVT in the presence of AD is challenging and poses the risk of further damage to vessels and other technical difficulties. However, the present cases demonstrated that EVT with minimal stenting and suction via the femoral route could be performed effectively and safely in patients with AD in a fashion similar to that of other hyperacute stroke patients.
Acknowledgements
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A6A1029617).
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
