 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(3); 2023 > Article |
|
Abstract
Developmental venous anomalies (DVAs) are composed of mature venous vessels that lack malformed or neoplastic elements. Although the hemorrhage risk is considered negligible, some patients may have neurological symptoms attributable to acute infarction or intracranial hemorrhage secondary to thrombosis, in the absence of a coexisting cavernous malformation. We report the case of a 42-year-old patient who presented with acute left-hand paresis secondary to a subcortical hemorrhage. This bleeding originated from a DVA in the corticospinal tract area and was surgically drained through an awake craniotomy. To accomplish this, we used a trans-precentral sulcus approach. After the complete removal of the coagulum, small venous channels appeared, which were coagulated. No associated cavernoma was found. Although the main DVA trunk was left patent, no signs of ischemia or venous infarction were observed after coagulating the small venous channels found inside the hematoma cavity. Two weeks after the procedure, the patient’s hand function improved, and he was able to resume desktop work. DVA-associated hemorrhage within the cortico-spinal tract could be safely removed with modern awake mapping techniques. This technique allowed the patient to rapidly improve his hand function.
Developmental venous anomalies (DVAs) are composed of mature venous vessels that lack malformed or neoplastic elements; they may be regarded as severe anatomic variants of medullary venous drainage [6]. These malformations are angiographically occult and were not consistently identified in the pre-magnetic resonance imaging (MRI) era [7]. Although the hemorrhage risk is considered negligible, some patients may have neurological symptoms attributable to acute infarction or intracranial hemorrhage (ICH) secondary to thrombosis in the absence of a coexisting cavernous malformation (CM). We report the case of a 42-year-old left-handed patient who presented with acute left-hand paresis secondary to a subcortical hemorrhage. This bleeding originated from a DVA in the corticospinal tract area and was surgically drained through an awake craniotomy. To accomplish this, we used a trans-precentral sulcus approach. No associated CM was found. Two weeks after the procedure, the patient’s hand function improved, and he was able to resume desktop work.
A left-handed 42-year-old male was referred to our department two weeks after the onset of left-hand weakness and clumsiness. The patient had no sensory deficits or weakness in the rest of his upper or lower limb muscles. To objectively assess his hand function, he underwent a coin rotation task [5], which showed impaired function (three coin flips in 10 seconds). This task is a validated bedside test that requires the participant to rotate a coin (the approximate size of a US nickel) through consecutive 180° turns, using the thumb and index, and middle fingers, as rapidly as possible for 10 seconds. Brain MRI showed a 4-cc right heterogeneous subcortical hemorrhage at the level of the corticospinal tract (CST), below the hand knob area. It had perilesional edema and signs of restriction in diffusion sequence. This lesion was predominantly hyperintense on T1 and hypointense with a hyperintense perilesional rim on T2-weighted images, which suggested acute bleeding. Notably, a DVA was observed adjacent to it on T2-weighted images (Fig. 1). Although no signs existed of an associated CM, we could not initially exclude it. Thus, with the possible coexistence of a CM in mind, we agreed with the patient and family to surgically remove the hematoma. The patient and his family were duly informed of the risks and benefits of the procedure and ultimately provided their consent.
The patient was placed in a supine position and secured awake in a Mayfield head holder with a previously described positioning technique used by our group since 2017 [1]. After sedation with dexmedetomidine and remifentanil, scalp infiltration was performed under local anesthesia. Optic neuronavigation (Tracker Navigation System, Física Médica SRL, Córdoba, Argentina) was introduced to aid in the design of the skin incision and plan the trajectory to the precentral sulcus. A right lazy S incision and frontoparietal craniotomy were performed (Fig. 2). After opening the dura, a fourchannel electrode strip was placed over the sensorimotor cortex to identify the central sulcus, precentral gyrus, and postcentral gyrus by recording the N20-P20 phase reversal somatosensory evoked potentials (SEPs). Once the precentral gyrus was identified, it was dissected under microscopic magnification. With the aid of neuronavigation and high-frequency monopolar cortical mapping, a small corticotomy was performed deep within the precentral sulcus. Entering the subcortical white matter, a firm coagulum was observed. This hemorrhage was carefully removed with suction. After the complete removal of the coagulum, small venous channels appeared, which were coagulated. Although the cavity was thoroughly explored, we could not find the main DVA trunk. Hemostasis was performed with oxidized regenerated cellulose and Surgiflo® (Surgiflo, Johnson & Johnson Wound Management, Somerville, NJ, USA). The dura mater and craniotomy were closed in a usual fashion. The patient was transferred to the intensive care unit, where he remained for 24 hours. Postoperatively, complete left-hand paresis was observed. Following a routine computed tomography that showed complete hemorrhage resection, he was transferred to the general ward and discharged 72 hours after the procedure. Serial neurological exams revealed progressive improvement of his hand function over the following days. Moreover, he was able to resume his office work two weeks after the procedure. Postoperative MRI was conducted eight months after the procedure, showing complete resection of the lesion, with sparing of the DVA (Fig. 3). No signs of ischemia were observed adjacent to the resection cavity. A thorough motor exam was performed 12 months after surgery, which showed frank improvement in his hand function (14 coin flips in 10 seconds in the coin rotation task).
DVAs are the most frequently identified intracranial vascular malformation (63% and 50% of all malformations in autopsy and MRI series, respectively) [3,12]. They have been hypothesized to result from a focal arrest of venous development and retention of primitive medullary veins that drain into a single, large vein [10]. The current medical literature indicates an increased prevalence of incidental DVAs, with a low ICH prevalence (ICH annual risk, 0.15% to 0.68%) and a low associated morbidity rate, as well as a negligible mortality rate [7,8]. Notably, DVAs have been associated with CMs in 8% to 33% of cases and together are the most common combination of mixed vascular malformations [9]. Although hemorrhage usually develops when a DVA is associated with a CM, ICH in the absence of an associated CM or arteriovenous malformation (AVM) does occur. One possible explanation is that ICH may destroy the architecture of a small underlying CM, which might not be detected even by pathological examination. In the present case, though no MRI or pathology signs existed of an associated CM, we could not exclude it.
Even though our patient showed a favorable outcome, controversy exists regarding optimal treatment for patients with hemorrhage within the perirolandic region. This region, also known as the central lobe [2] or paracentral area [13], is one of the most eloquent areas of the brain. When operating in the perirolandic region, remaining aware of the arterial supply and venous drainage is important since neurological deficits following tumor resection are more frequently due to arterial or venous infarction than to cortical or subcortical structural impairment [12]. In our case, despite the known risks, we agreed with the patient and family to surgically drain the hemorrhage to reduce the biochemical and inflammatory processes initiated by toxic blood products. Hence, the patient’s hand function improved over a few days. Despite the close relationship with the CST (which was spread out by the hemorrhage), approaching the lesion via the precentral sulcus proved a valuable strategy to avoid new neurological deficits. This strategy was used also by Sanmillan et al. [11] to perform resections of brain metastasis located immediately anterior to the CST. Finally, despite the coagulation of small venous channels inside the hematoma cavity, no signs of ischemia or venous infarction were observed postoperatively.
DVA-associated hemorrhage within the CST could be safely removed with modern awake mapping techniques. This technique allowed the patient to rapidly improve his hand function. Small venous channels found inside the hematoma cavity could be coagulated without signs of ischemia or venous infarction.
ACKNOWLEDGEMENTS
The authors would like to thank Maria Fernanda Ruiz for her assistance in preparing the pathology section.
Fig. 1.
(A) Axial T2-weighted magnetic resonance imaging (MRI) revealing a 4-cc hypointense lesion. An irregular hyperintense perilesional rim was observed. This lesion was located subcortically, within the corticospinal tract. (B) Axial T2-weighted MRI showing edema at the level of the hand knob. (C) Sagittal non-contrast T1-weighted MRI revealing a hyperintense mass. (D) Coronal SWI-weighted MRI. A developmental venous anomaly is visualized adjacent to the hemorrhage. (E and F) MRI tractography of the corticospinal tract. Note that the hand fibers were spread out by the hemorrhage.
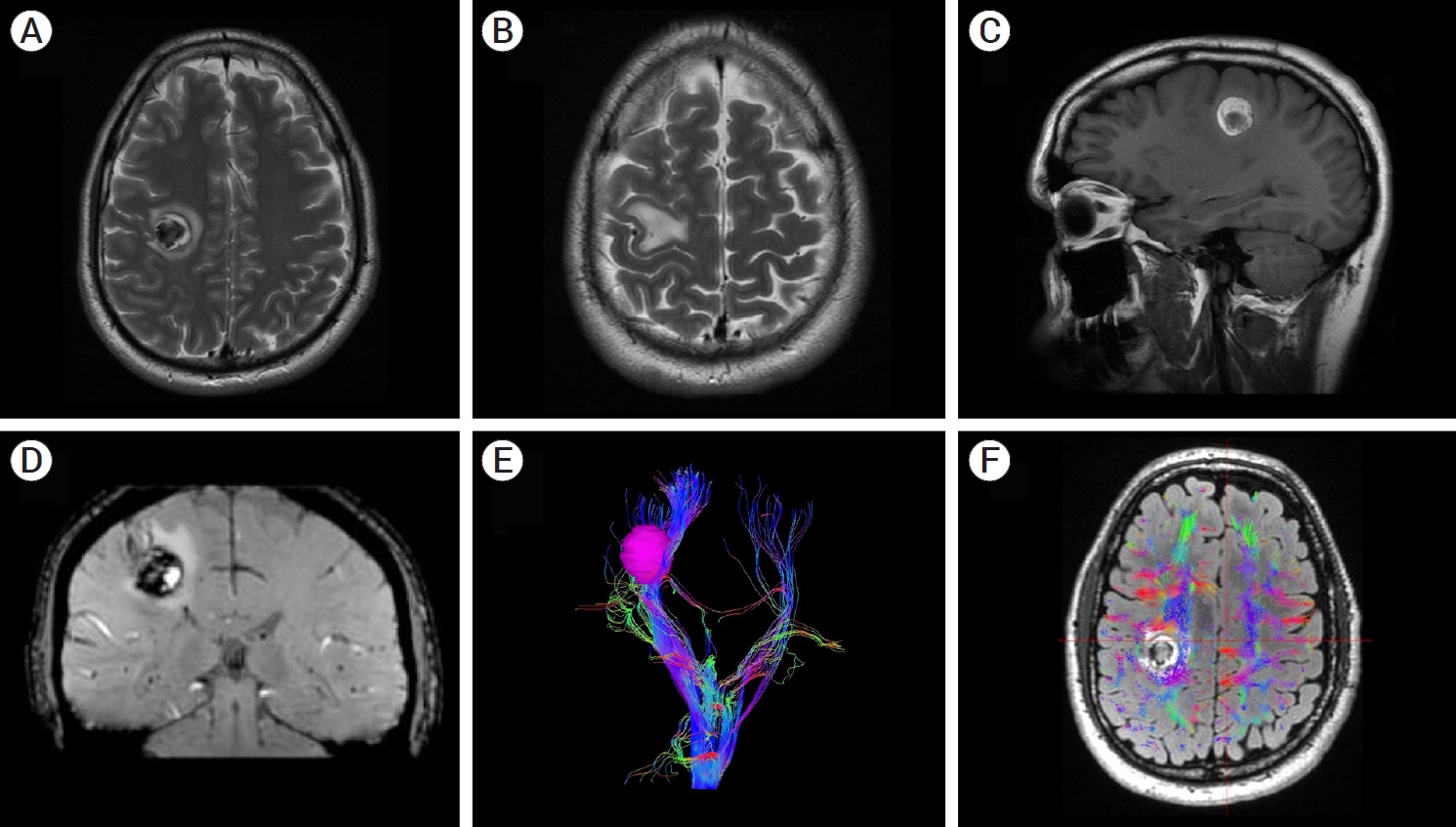
Fig. 2.
The patient was operated on awake. (A) Initial step with a nasal cannula. EMG electrodes were placed in his upper and lower extremities. (B) Intraoperative photograph showing the planned craniotomy. A right lazy S incision and frontoparietal craniotomy were performed. (C) Microscopic photograph showing the initial cortical exposure. Note the hand knob (black star) and precentral sulcus (white arrow). (D and E) High-frequency monopolar cortical mapping of the precentral gyrus and sulcus. (F) Exposure of the hemorrhage after opening the precentral sulcus. Several venous channels (white arrow) were found and thoroughly coagulated within the hematoma. No cavernous malformation was found within or adjacent to the hematoma cavity. (G) On hematoxylin and eosin sections, there are small to medium-sized blood vessels and macrophages containing hemosiderin pigment (40×).
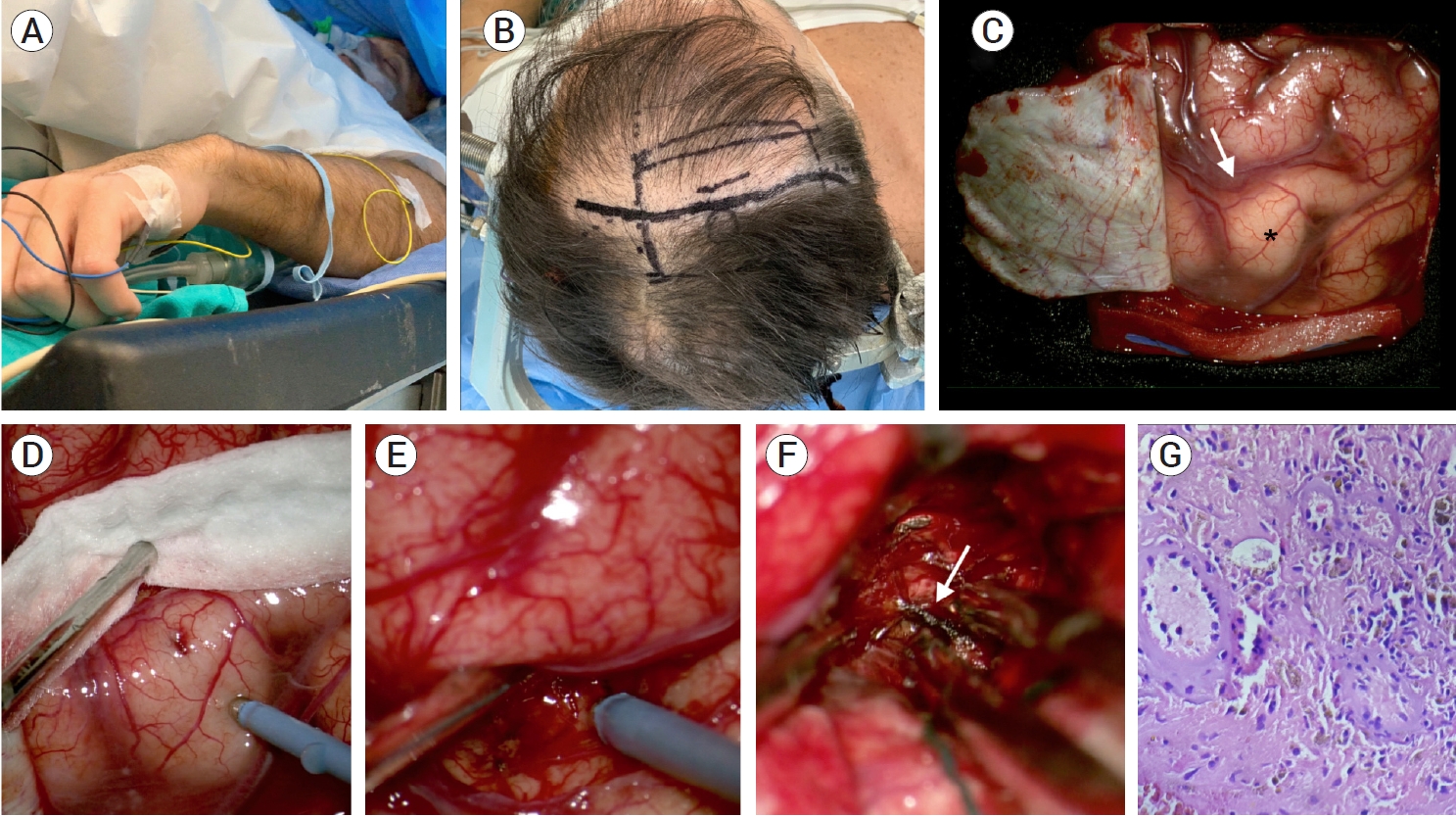
Fig. 3.
(A) Eight-month axial T2-weighted magnetic resonance imaging (MRI) showing complete resolution of the hemorrhage. (B) Axial T2-weighted MRI at the level of the hand knob. Note mild atrophy of this cortical area. (C and D) MRI tractography of the corticospinal tract. Note the proximity of the resection cavity (white arrow) with this structure. (E) Coronal T1-weighted contrast-enhanced MRI. The main developmental venous anomaly remains patent (white arrow).

REFERENCES
1. Barrenechea IJ, Rojas H, Nicola M, Marquez L, Herrera R, Van Isseldyk F. A novel temporary cranial fixation device for awake cranial surgery: technical report of 14 cases. Surg Neurol Int. 2020 Jan;11:12.



2. Frigeri T, Paglioli E, de Oliveira E, Rhoton AL Jr. Microsurgical anatomy of the central lobe. J Neurosurg. 2015 Mar;122(3):483-98.


3. Garner TB, Del Curling O Jr, Kelly DL Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg. 1991 Nov;75(5):715-22.


4. Gempt J, Krieg SM, Hüttinger S, Buchmann N, Ryang YM, Shiban E, et al. Postoperative ischemic changes after glioma resection identified by diffusion-weighted magnetic resonance imaging and their association with intraoperative motor evoked potentials. J Neurosurg. 2013 Oct;119(4):829-36.


5. Hill BD, Barkemeyer CA, Jones GN, Santa Maria MP, Minor KS, Browndyke JN. Validation of the coin rotation test: a simple, inexpensive, and convenient screening tool for impaired psychomotor processing speed. Neurologist. 2010 16(4):249-53.


6. Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev. 1986 Sep;9(3):233-42.



7. McLaughlin MR, Kondziolka D, Flickinger JC, Lunsford S, Lunsford LD. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998 Aug;43(2):195-200; discussion 200-1.


8. Naff NJ, Wemmer J, Hoenig-Rigamonti K, Rigamonti DR. A longitudinal study of patients with venous malformations: documentation of a negligible hemorrhage risk and benign natural history. Neurology. 1998 Jun;50(6):1709-14.


9. Rabinov JD. Diagnostic imaging of angiographically occult vascular malformations. Neurosurg Clin N Am. 1999 Jul;10(3):419-32.


10. Saito Y, Kobayashi N. Cerebral venous angiomas: clinical evaluation and possible etiology. Radiology. 1981 Apr;139(1):87-94.


- TOOLS
-
METRICS

-
- 2 Crossref
- 0 Scopus
- 1,246 View
- 29 Download
- ORCID iDs
-
Ignacio J. Barrenechea

https://orcid.org/0000-0002-6964-9110 - Related articles
-
Intracranial Pial Arteriovenous Fistula Presenting with Hemorrhage: A Case Report2012 December;14(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



