Parent artery occlusion of a giant internal carotid artery pseudoaneurysm-related direct carotid cavernous fistula: A case report
Article information
Abstract
Traumatic internal carotid artery injuries can produce direct carotid-cavernous fistulas as well as giant internal carotid artery pseudoaneurysms. Clinical sequelae can include headaches, cranial nerves palsies, proptosis, chemosis and optic neuropathy with visual loss as the most dangerous complication. Herein, we present a case of one of the largest reported internal carotid artery pseudoaneurysms associated with a direct carotid cavernous fistula. We describe the techniques and pitfalls of treatment with parent vessel occlusion.
INTRODUCTION
A carotid cavernous fistula (CCF) is an arteriovenous shunt into the cavernous sinus from either internal carotid artery (ICA) and/or external carotid artery (ECA) and their branches [10]. A direct carotid cavernous fistula represents a defect in the wall of the cavernous ICA with shunting of blood flow into veins of the cavernous sinus, and may be caused by trauma, rupture of a cavernous ICA aneurysm, or spontaneous formation in the setting of connective tissue disease. Conversely, an indirect carotid cavernous fistula is a dural arteriovenous fistula in the region of the cavernous sinus, and may be supplied by dural branches of the ECA, ICA, or both.
CCFs are classified based on the direction of flow and arterial anatomy with Barrow classification being the most widely adopted ranking system. However, there are limitations of this classification system in clinical practice as treatment approach is mostly based on venous rather than arterial architecture of the fistulas. Thomas et al. proposed an updated classification, which is based on the routes of venous drainage and correlates with symptomatology and preferred treatment approach [10].
In rare cases, a direct CCF may be associated with a giant ICA pseudoaneurysm (size more than 25 mm), which can exert significant mass effect on surrounding structures, including cranial nerves, orbit, and brain [3]. Although a vessel-preserving approach is preferred when treating giant internal carotid artery pseudoaneurysms, this is not always possible and parent vessel occlusion may be necessary.
This report demonstrates an unusual case of a longstanding giant ICA pseudoaneurysm and direct CCF that resulted in temporal lobe compression, proptosis and sixth nerve palsy, and successful treatment with parent vessel occlusion.
CASE DESCRIPTION
A 38-year-old Spanish-speaking male non-smoker presented with new right-sided headache associated with right eye pain. His only prior medical history was a severe traumatic brain injury secondary to falling off a horse 12 years prior, which was complicated by prolonged coma and right abducens palsy. Further details regarding this injury were not available as this event occurred in another country. He denied any history of significant headache until several months prior to his current presentation. An MRI and MRA of the brain showed a 3.7×3.7×4.6 cm right cavernous ICA pseudoaneurysm extending into the middle cranial fossa with profound mass effect on the adjacent temporal lobe (Fig. 1). Examination at that time was notable for complete right abducens palsy and 5 mm proptosis of the right eye.
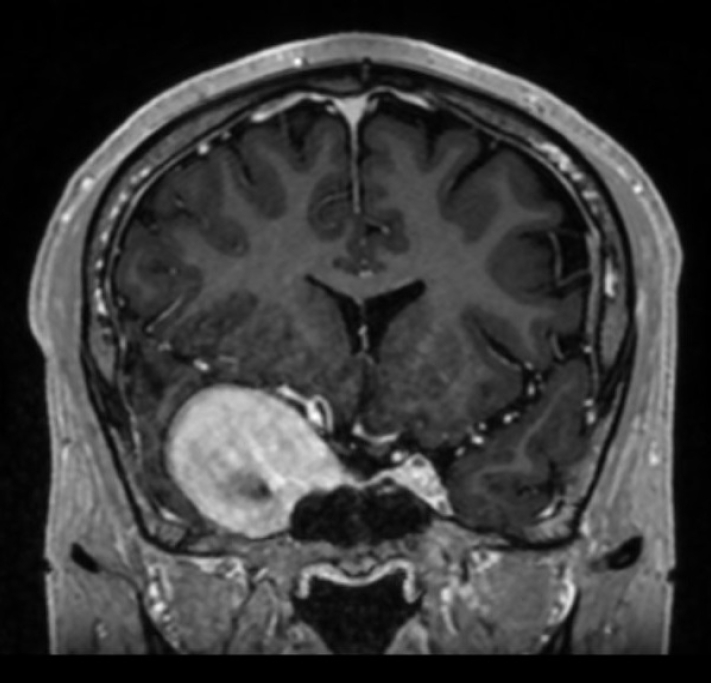
Initial MRI demonstrating giant right internal carotid artery pseudoaneurysm with severe mass effect on the adjacent temporal lobe, and effacement of the right lateral ventricle. MPRAGE, coronal view.
A cerebral angiogram demonstrated a giant right cavernous segment ICA aneurysm measuring 5.7×4.7 cm, and shunting into the cavernous sinus consistent with a direct CCF (Fig. 2A). Venous drainage was via the inferior petrosal sinus without evidence of cortical venous drainage. There was no flow to the brain on the right ICA injection and in fact no visible flow beyond the cavernous segment (Fig. 2A). The right ICA was reconstituted by the posterior communicating artery (Fig. 3). Because there no visible outflow vessel from the pseudoaneurysm and angiographically demonstrated adequate collateral supply, we chose to treat the pseudoaneurysm with parent vessel occlusion rather than a vessel-preserving technique such as flow diversion or covered stent placement. Eight coils were deployed via a PX Slim microcatheter (Alameda, CA, USA) followed by an Amplatzer plug (Chicago, IL, USA) to occlude the cervical and petrosal segments of the right ICA. Despite successful right ICA occlusion, there was continued filling of the pseudoaneurysm and CCF from branches of the ECA, including the ethmoidal arteries to the ophthalmic artery and the right middle meningeal artery (Fig. 2B). This finding was consistent with continued filling of the aneurysmal cavernous segment of the ICA via artery to artery collaterals, which were kept open by the high flow arteriovenous shunt. The patient was started on aspirin 81 mg after vessel occlusion to decrease the risk of thromboembolic complications. An angiogram was repeated in one month to assess the CCF and pseudoaneurysm, and it demonstrated persistent filling of the aneurysm mostly via the right middle meningeal artery (Fig. 2C).
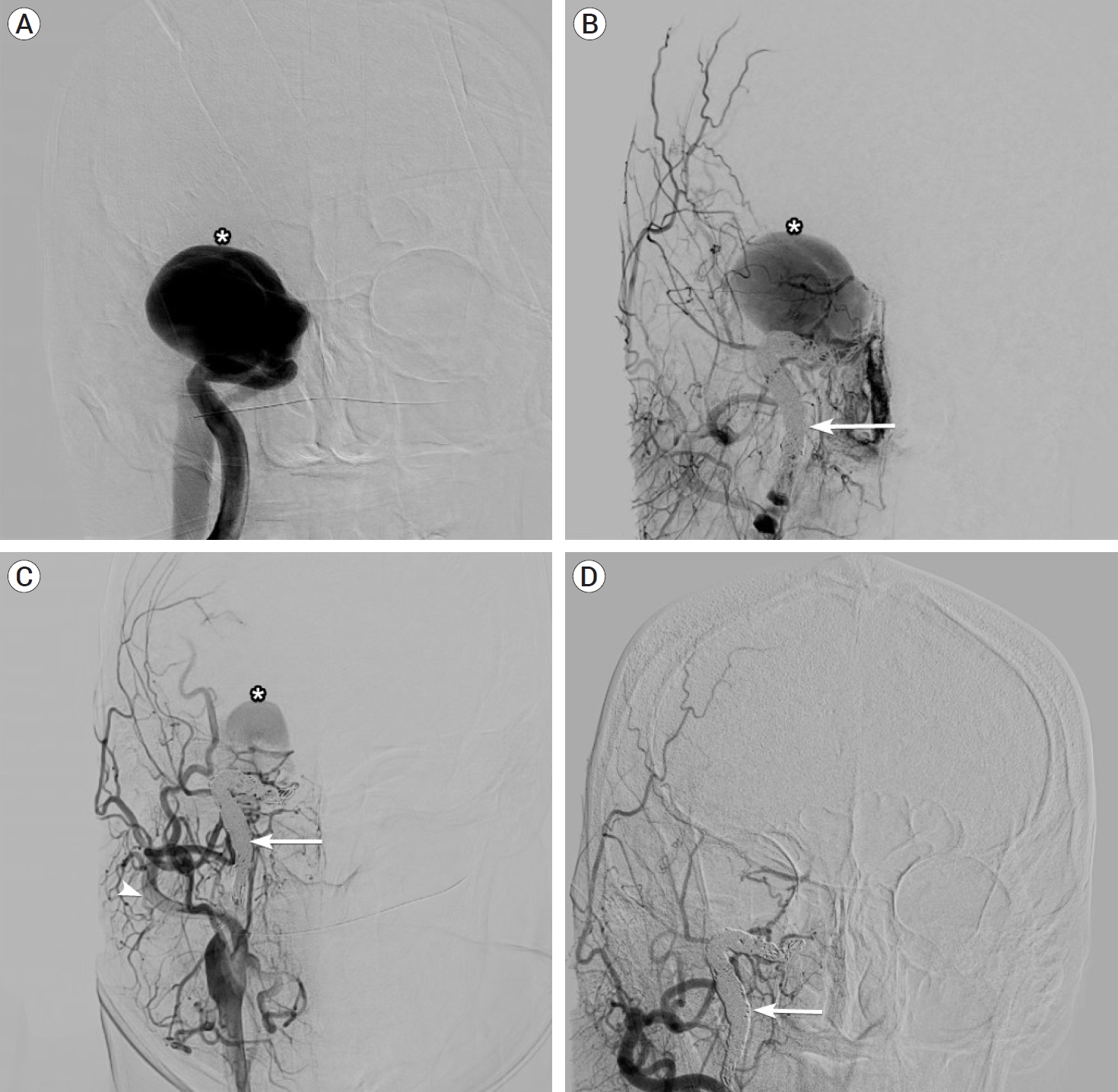
(A) Initial right ICA angiogram demonstrating giant carotid pseudoaneurysm and direct CCF (asterisk) with no visible flow beyond the cavernous segment. (B) Right CCA angiography following coiling and occlusion of the ICA (arrow) with persistent pseudoaneurysm filling. (C) Right CCA (arrowhead) angiogram at one month demonstrating residual filling of pseudoaneurysm via external carotid artery collaterals. (D) Final ECA angiogram demonstrating spontaneous occlusion of the pseudoaneurysm. ICA, internal carotid artery; CCF, carotid cavernous fistula; CCA, common carotid artery; ECA, external carotid artery
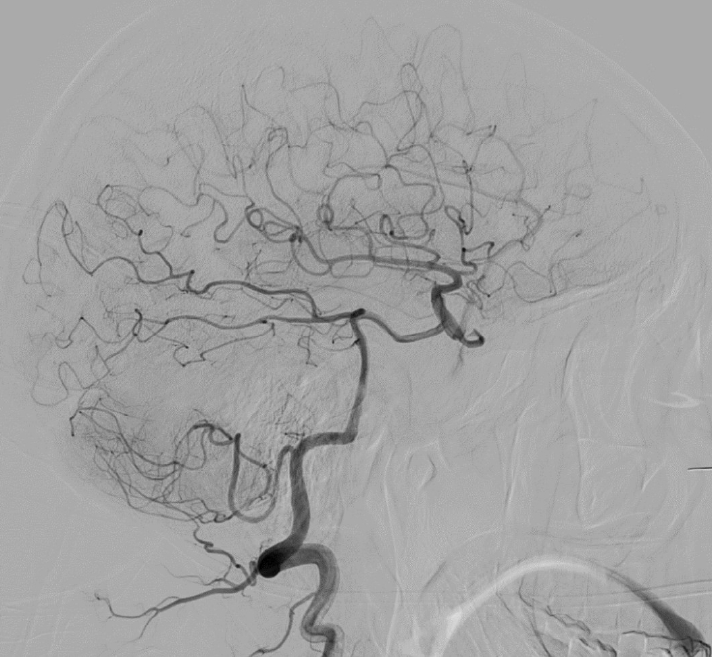
Initial right vertebral artery angiogram demonstrating reconstitution of the ICA by the posterior communicating artery. ICA, internal carotid artery
Five days after the follow up angiogram, the patient presented to the emergency department with intractable headache, right eye pain, nausea and vomiting. Detailed ophthalmological and neurological exams did not reveal any meaningful change from his baseline exam. CT angiogram of the head showed absence of arterial phase filling into the pseudoaneurysm. Repeat angiography no longer showed residual flow into the fistula (Fig. 2D), consistent with spontaneous thrombosis. He was treated with a steroid taper for his pain and was discharged from the hospital next day with close neurosurgery follow-up.
DISCUSSION
Intracranial ICA pseudoaneurysms from blunt head trauma are rare [2]. Furthermore, the combination of traumatic CCF and giant ICA pseudoaneurysm is exceptionally rare and has only been described in a few case reports [4,8].
CCFs are classified as direct or indirect and are further classified based on their venous drainage. Direct CCFs result from direct connection between the ICA and cavernous sinus and are usually high flow. Indirect fistulas result from indirect communication between the cavernous sinus and ICA and/or ECA via dural arterial branches, and usually have low-flow physiology [1]. Although this case featured filling of the pseudoaneurysm and CCF from ECA branches, this does not represent a simultaneous indirect CCF but rather “sumping” of flow from artery to artery collaterals after proximal ICA occlusion in the setting of a high flow shunt (Fig. 2A, C).
At 5.7 cm in diameter, this report demonstrates one of the largest reported ICA aneurysms or pseudoaneurysms to our knowledge. Prior reports have cited aneurysmal diameter 3.7×4.5 cm in the carotid segment ICA [5]. Thus, it is not surprising that no other reports to our knowledge have described the significant aneurysmal mass effect seen in this patient, with displacement of the right temporal lobe and effacement of the ventricle.
Most CCFs present with neuroophthalmological complaints from orbital venous hypertension which may cause cranial nerves palsies, optic neuropathy, proptosis and chemosis. As with dural arteriovenous fistulas at other sites in the nervous system, the symptomatology is dictated by the venous drainage. Symptoms may also be produced by direct mass effect on the cavernous sinus [6]. Direct fistulas tend to present more acutely and progress rapidly, whereas indirect fistulas tend to have a more indolent course [9]. In our case, the CCF and associated pseudoaneurysm developed secondary to remote blunt trauma, and the relatively long delay to diagnosis likely contributed to the development of a high flow fistula with a “functionally occluded” ICA. Focal neurologic signs or symptoms consistent with right temporal lobe dysfunction (e.g. seizure) were never reported or observed despite significant compression. However, non-dominant temporal lobe lesions can produce subtle, nonspecific symptoms that are easily missed, such as anxiety, depression, rage attacks, visuospatial amnesia and receptive amusia. Thus, it is difficult to appreciate whether this patient had temporal lobe dysfunction in the absence of a detailed neuropsychological evaluation [7]. It is possible that his worsening headache was the first symptom of temporal lobe compression, and without appropriate treatment, the patient may have developed life threatening seizures and/or strokes.
Parent vessel occlusion was once a common treatment for giant aneurysms of the ICA although it has largely been supplanted by flow diversion, which allows preservation of the vessel and reconstruction of the lumen. However, parent vessel occlusion may still be required in certain cases. In this case, the absence of a visible outflow vessel precluded flow diversion and the presence of complete collateralization from the posterior communicating artery allowed parent vessel occlusion to be performed. Interestingly, occlusion of the ICA proximal to the lesion was met with continued filling of the pseudoaneurysm and CCF from artery to artery collaterals from the middle meningeal artery and ethmoidal arteries (via the ophthalmic artery). This may have been prevented by starting the coiling process at the level of the pseudoaneurysm itself; however, this would have been expected to produce mass effect in the cavernous sinus which would have been contrary to the goal of treatment. Fortunately, the small collaterals filling the lesion spontaneously occluded over time.
CONCLUSIONS
A delayed diagnosis of cerebrovascular injury can lead to the formation of a giant cavernous ICA pseudoaneurysm, which can present with mass effect on the cavernous sinus and ipsilateral temporal lobe. An associated direct CCF may also be present. Although parent vessel occlusion is an effective treatment, proximal vessel occlusion may initially not cure the lesion due to collateral filling.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
