 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(2); 2023 > Article |
|
Abstract
Embolization of the middle meningeal artery (MMA) is a safe and effective adjunct in the treatment of chronic subdural hematoma. While prior authors describe the use of coils to assist embolization by preventing reflux through eloquent collaterals, we de- scribe the use of coils to further open the MMA, allowing the administration of greater amounts of embolisate for a more robust embolization. The objective of this study was to demonstrate that helical coils can safely open the MMA following the administration of polyvinyl alcohol (PVA) particles. This allows for more embolisate to be administered into the MMA for more effective treatment. A retrospective review was conducted at our institution including intraoperative images and postoperative clinical and radiographic follow up. Failure rates using MMA embolization with PVA and helical coil augmentation were compared to failure rates in the literature of MMA embolization with PVA or ethylene vinyl-alcohol copolymer alone. A total of 8 cases were reviewed in which this technique was implemented. There were no immediate complications after treatment. All patients that underwent helical coil embolization following the administration of PVA had increased amount of embolisate delivered into the MMA. All patients at follow up had resolution of the subdural hematoma on outpatient imaging. Helical coil embolization allows for more embolisate administration into the MMA and provides a technical advantage for patients that fail traditional techniques of embolization. Case series are taking place to further test this hypothesis and identify the ideal patient population that may gain maximal yield from this novel technique.
Chronic subdural hematoma (cSDH) is a common neurosurgical pathology that occurs often in elderly patients due to increased fall risk and reduced brain volume [31,32,36]. Although commonly traumatic, cSDH may occur spontaneously, especially in the setting of intrinsic coagulopathy or anticoagulant medication use [35]. As the population ages and anticoagulant use increases, the incidence of cSDH has risen, and is predicted to become the most common neurosurgical pathology by the year 2030.1) Standard treatments for cSDH include twist-drill craniotomy, burr hole craniotomy, or mini-craniotomy for hematoma evacuation [4,14,34], fenestration of subdural membranes [4], and placement of a closed drainage system postoperatively to evacuate any remaining blood products or pneumocephalus [26,30]. However, symptomatic recurrence rates ranging from 5-30% have prompted efforts to devise novel treatments for this disease process [6,13,17,28,31]. In particular, middle meningeal artery (MMA) embolization has recently been demonstrated to be a safe and effective adjunctive treatment for cSDH with low complication and recurrence rates, and a high rate of hematoma resolution [3,14,19,25].
The rationale for MMA embolization is derived from the pathophysiology of cSDH.
Accumulation of blood in the subdural space triggers a local inflammatory reaction, which leads to proliferation of fibroblasts, formation of granulation tissue, and release of inflammatory and angiogenic factors [7]. This inflammatory cascade results in the formation of inner and outer neomembranes surrounding the growing hematoma [7]. Angiogenic mediators (i.e., VEGF) promote the growth of fragile, leaky capillaries along the outer subdural membrane, which extravasate blood and contribute to hematoma formation and recurrence after surgical evacuation [7,11,32]. As the primary blood supply to the outer neomembrane is the MMA, embolization of the MMA obliterates the dural neovasculature preventing further blood extravasation and hematoma recollection [7,23,25]. Further support for this hypothesis includes studies demonstrating larger caliber MMAs in patients with cSDH [31], wispy appearance of distal MMA branches on angiography (often referred to as “cotton-like blushing” likely representing dural membrane neovasculature) [10,14], and contrast staining of the membranes surrounding the subdural space on computed tomography (CT) after angiography [20].
To achieve optimal MMA embolization for cSDH, it is critical that embolisate reaches the distal vessels feeding the outer neomembrane while avoiding embolisate reflux and penetration into dangerous MMA collaterals to the orbit (i.e., the lacrimal branch), or feeding cranial nerves (i.e., the petrosal branch, which feeds the vasa nervorum of the facial nerve) [3,15,27]. While prior authors describe the use of coils to prevent reflux through collateral vessels [22] or to block distal flow of embolisate [15], we describe the first use of helical coils to further open the MMA during embolization, such that a greater quantity of embolisate is administered to the distal vasculature, resulting in a more robust embolization. This technique is graphically represented in Fig. 1. We compared our results to the existing published failure rates within the literature.
Institutional review board (IRB) approval was obtained (IRB No. 210854) for a retrospective review of all patients who underwent MMA embolization with helical coiling from January 2021 to July 2021 at a single institution. As this was a retrospective chart review, patient consent was not required. Thorough discussions explaining risks, benefits, and alternatives of the procedure were held with each patient, and all patients consented to the procedure. The patients included in this study were not consecutive and were treated in a single sitting. The minimum follow-up time was 3 months. Follow up results were both clinical stability or improvement and radiographic improvement of the cSDH obtained by non-contrast CT scan of the head. The indications for MMA embolization with helical coil augmentation were as follows: cSDH causing clinical symptoms or midline shift, or recurrence after prior surgical evacuation. Exclusion criteria included subdural hematoma in the setting of an underlying lesion (i.e., vascular abnormality or tumor), focal, nonconvexity cSDH where the MMA was unlikely to be the primary vascular supply, cSDH causing significant midline shift and neurologic deficit requiring surgical decompression, and significant thrombocytopenia (platelets <75k/µL). Outcomes recorded included postoperative cranial neuropathies, new neurologic deficits, hematoma recurrence, change in hematoma size on routine follow-up imaging and complications. Intraoperative images as well as postoperative clinical and radiographic follow-up were reviewed. We compared these results to the reported failure rate of MMA embolization in the literature to serve as a retrospective control group. The PubMed database was searched for articles between January 2018 and January 2022. The search terms utilized were (“middle meningeal artery embolization”) AND (“chronic subdural hematoma”). To determine a failure rate in our study, radiographic change in SDH size (measured in millimeters) at initial encounter and at 3 month follow up was assessed. A comparison was made between success of treatment in MMA embolization alone and MMA embolization using this novel technique. Success of treatment was defined as a reduction in radiographic hematoma size at follow up compared to pre-MMA embolization baseline status, while failure was defined as any radiographic increase in SDH size or neurological deficit after the procedure.
All MMA embolizations were performed in the interventional radiology biplane angiography suite. A combination of either general anesthesia or moderate sedation was used. Endovascular access was obtained via the femoral artery in all patients, and the embolisate material used was highly diluted 150-250 micron polyvinyl alcohol (PVA) particles in Omnipaque 240. Microcatheters utilized were either the EXCELSIOR SL10 (Stryker, Kalamazoo, MI, USA) or ECHELON microcatheters (Medtronic, Dublin, Ireland). After femoral access was obtained, the guide catheter was advanced to the proximal external carotid or distal common carotid artery. The microcatheter was advanced into the MMA, proximal to its bifurcation into anterior and posterior branches, for superselective angiography (Fig. 2A). Collateral vessels were identified prior to embolization, focusing on the lacrimal and petrous branches, and particular care was taken to verify that the MMA did not originate from the ophthalmic artery. The anterior and posterior MMA branches were accessed, PVA was deployed into each until flow stasis occurred, and superselective angiography was repeated (Fig. 2B). Then, the helical coils were deployed demonstrating reconstitution of the MMA (Fig. 2C), followed by repeated injection PVA, and final angiogram demonstrating complete obliteration of the vessel (Fig. 2D). Common carotid artery angiography was performed to ensure patency of all intracranial vessels. The catheters were then withdrawn, a final angiogram of the femoral artery was obtained to evaluate for injury, and closure was performed using the Angio-Seal closure device (Terumo Medical Corp., Somerset, NJ, USA) and/or manual pressure. Post-operative CT was obtained within 24 hours and on subsequent follow-up visits as per standard protocol.
Baseline characteristics of the eight patients who underwent MMA embolization with helical coil augmentation are displayed in Table 1. Intraoperative angiographic imaging is demonstrated in Fig. 2. This indicates the MMA prior to treatment (Fig. 2A), initial PVA embolization (Fig. 2B), augmentation of blood flow in the MMA after deployment of helical coils (Fig. 2C), and complete obliteration of the MMA after the second deployment of embolisate (Fig. 2D). In the immediate postoperative period, there were no neurologic or cranial nerve deficits. At 3-month follow-up, there have been no symptomatic recurrences and no re-operations in this cohort. All patients demonstrated stable or reduced hematoma size at last follow-up. One out of 8 patients (13%) included in this cohort underwent standard surgical intervention prior to the MMA embolization given mass effect. Embolization was utilized as an adjuvant treatment in these cases for a more durable treatment. Fig. 3 demonstrates a marked reduction in hematoma size at 4 months follow up in a patient that underwent helical augmented PVA embolization of the MMA.
In 2019, Link et al. demonstrated a failure rate of 8.0% in a series of 60 patients who underwent MMA embolization. In other words, 92% of patients in that study were able to avoid surgery after MMA embolization [19]. A recent systematic review and meta-analysis by Ironside et al. describing pooled data from 20 studies on MMA embolization for cSDH reported a hematoma recurrence rate of 4.8% for MMA embolization compared to 21.5% for surgical intervention (OR 0.15, 95% CI 0.03-0.75; p=0.02) [12]. Kim et al. similarly reported a failure rate of 3.8% in patients who underwent MMA embolization for refractory cSDH [16]. In another study including 541 patients, Ban et al. compared standard treatment options with MMA embolization; treatment failure was found in 1.4% of patients treated with MMA embolization [2]. In this study, 8 patients underwent MMA embolization with helical coil augmentation. A radiographic reduction in SDH size at 3-month follow up was seen in 6 patients2 patients are now deceased due to causes unrelated to the procedure or cSDH. One patient suffered cardiac arrest due to severe baseline cardiopulmonary disease and cardiopulmonary resuscitation was not successful. The other patient succumbed to toxic metabolic encephalopathy due to uncontrolled diabetes mellitus. Out of the 6 patients evaluated at 3 month follow up, 6 had reduced SDH sizes, demonstrating a 100% success of treatment. In other words, thus far, no patients have failed treatment using this novel technique.
In this technical case series, we described a novel technique by which a helical coil was employed to augment blood flow through the MMA and improve penetration of embolisate to the distal branches feeding the outer dural membrane in eight patients with chronic subdural hematoma. This technique allowed for greater amounts of embolisate to be delivered to distal vasculature resulting in a more robust embolization. We report no new neurological deficits, no cranial neuropathies, and no hematoma recurrence at the latest follow-up within six of these cases. While adjunctive helical coils have been used in glue embolization (n-Butyl Cyanoacrylate, n-BCA) of vein of Galen malformations to reduce arterial flow velocity, improve control while deploying the glue, and providing mechanical stabilization within the artery [22], there are no prior reports describing this strategy in the context of MMA embolization to increase penetration of PVA. We selected helical coils as they are ideal for achieving tight packing in small vessels such as the MMA. The outward radial force of the helical coil, which can be sized specifically to the luminal diameter of the vessel, functions like a stent to maintain the vessel diameter and optimize distal penetration of particles.
MMA embolization has transformed the care for patients with cSDH [2,9,18,19] as either routine prophylaxis to reduce to the risk of cSDH recurrence or progression, or as an adjunct to surgical evacuation [14]. The excellent outcomes in these studies have prompted several retrospective cohort studies [2,16,21,29] and one prospective cohort study [24] comparing MMA embolization with observation or surgical evacuation. Specifically, the largest retrospective cohort study was published by Shotar et al. and compared 89 patients treated with prophylactic MMA embolization after surgical evacuation against 174 patients treated with surgical evacuation alone [29]. They found significantly lower recurrence rate in the embolization group compared to the control (4% vs. 14%; Odds Ratio [OR] 0.28, 95% confidence interval [CI] 0.07-0.86; p=0.02). The prospective study by Ng et al. randomized 46 patients to surgery alone vs. surgery with MMA embolization and found greater hematoma volume resorption in the embolization group at 3 months follow-up (mean difference 17.5 mL, 95% CI 3.87-3.36 mL; p=0.015), with no difference in rate of recurrence (5.3% vs. 4.5%) [24]. These excellent outcomes have stimulated further interest into MMA embolization within the neurosurgical community, and as a result there are 11 prospective randomized trials currently recruiting patients globally [5]. Compared to the reported failure rates in the literature, the technique described in this study has demonstrated 100% efficacy since no patients failed treatment (Table 2).
As MMA embolization has grown in popularity, there has been debate regarding the optimal choice of embolic agent (coils, polyvinyl alcohol particles, n-BCA, or ethyl vinyl alcohol copolymer), optimal site of vascular access (transfemoral or transradial), patient selection, and timing of the procedure [8]. Recently, a single-institution study by Catapano et al. retrospectively compared different methods of MMA embolization for cSDH in 35 patients (41 hematomas). The authors found no significant differences in hematoma resolution rate among different embolisates used (Onyx 69%, n-BCA 50%, p=0.25), findings replicated in a larger (138 subjects) prospective multicenter series by Kan et al. (mean SDH reduction percentage: coils 48.9%, liquid embolic 63.1%, particles 58.2%; p>0.05). Neither study found differences in outcomes between transfemoral and transradial access, although Catapano et al. reported a preference for transradial access to mitigate the risk of life-threatening retroperitoneal hematoma that may occur with transfemoral access [3]. In terms of MMA embolization technique, Catapano et al. found that embolization of the anterior and posterior MMA branches was associated with a higher rate of complete resolution (76% vs. 33%, p=0.01) [3]. While Kan et al. did not stratify by embolization technique, they did report a low symptomatic recurrence rate (6.3%) and low reoperation rate (6.5%) [14]. Notably, this cohort included patients with cSDH that were unilateral or bilateral, first-occurrence or recurrent, and many of whom were taking antithrombotic or anticoagulant medications, which implies the indications for MMA embolization are broad.
The degree by which embolisate penetrates distal capillaries closest to the dural membrane may be associated with improved embolization result [20,27]. Rutledge et al. described a case in which tortuosity of the MMA prevented the authors from embolizing the MMA distally; at one month postoperative, an interval head CT demonstrated a large increase in the hematoma size with increased midline shift requiring a second procedure [27]. Helical coil augmentation described in the present study could have improved distal embolisate delivery and prevented hematoma growth. On the other hand, the study by Catapano et al. compared distal and proximal with proximal only embolization and found no significant differences in change in hematoma size (13.2 cm vs. 11.4 cm; p=0.36), percentage of procedures with complete resolution (64% vs. 63%; p=0.92), or percentage with treatment failure (0% vs. 6%, p=0.21) [3]. However, the sample size was small (25 distal and proximal, 16 proximal only), and may have been underpowered. Regardless, in the present study, adjunctive treatment with helical coils increased the patency of the MMA resulting in greater embolisate delivery to the distal branches and achieved successful and robust embolization in all eight subjects. While this preliminary series supports the safety and efficacy of helical coil augmentation, future work involving larger prospective trials is required to make more definitive statements regarding circumstances under which helical coil augmentation is preferred.
We acknowledge that this is a small case series to demonstrate a concept. A larger patient sample would render our findings more conclusive. Additionally, the absence of a failure rate using this novel technique makes further investigation necessary to continue to prove its safety and efficacy.
Preventing recurrence of the highly prevalent chronic subdural hematoma remains a challenge within the neurosurgical community. Embolization of the middle meningeal artery attempts to address the root cause of cSDH; obliteration of neovasculature feeding the dural membrane may prevent recollection of the hematoma. This case series is the first demonstration of the safety and efficacy of the deployment of a helical coil to improve delivery of polyvinyl alcohol particle embolisate to the distal neovasculature of the MMA, creating a more robust embolization. We found no cranial neuropathies, no new neurological deficits, and no symptomatic or radiographic recurrences at the most recent follow up. Further comparative studies are required to validate and further delineate the role of helical coils as an adjunct to MMA embolization in the treatment of cSDH.
Fig. 1.
Graphic representation of helical coil technique for MMA embolization. PVA, polyvinyl alcohol; MMA, middle meningeal artery
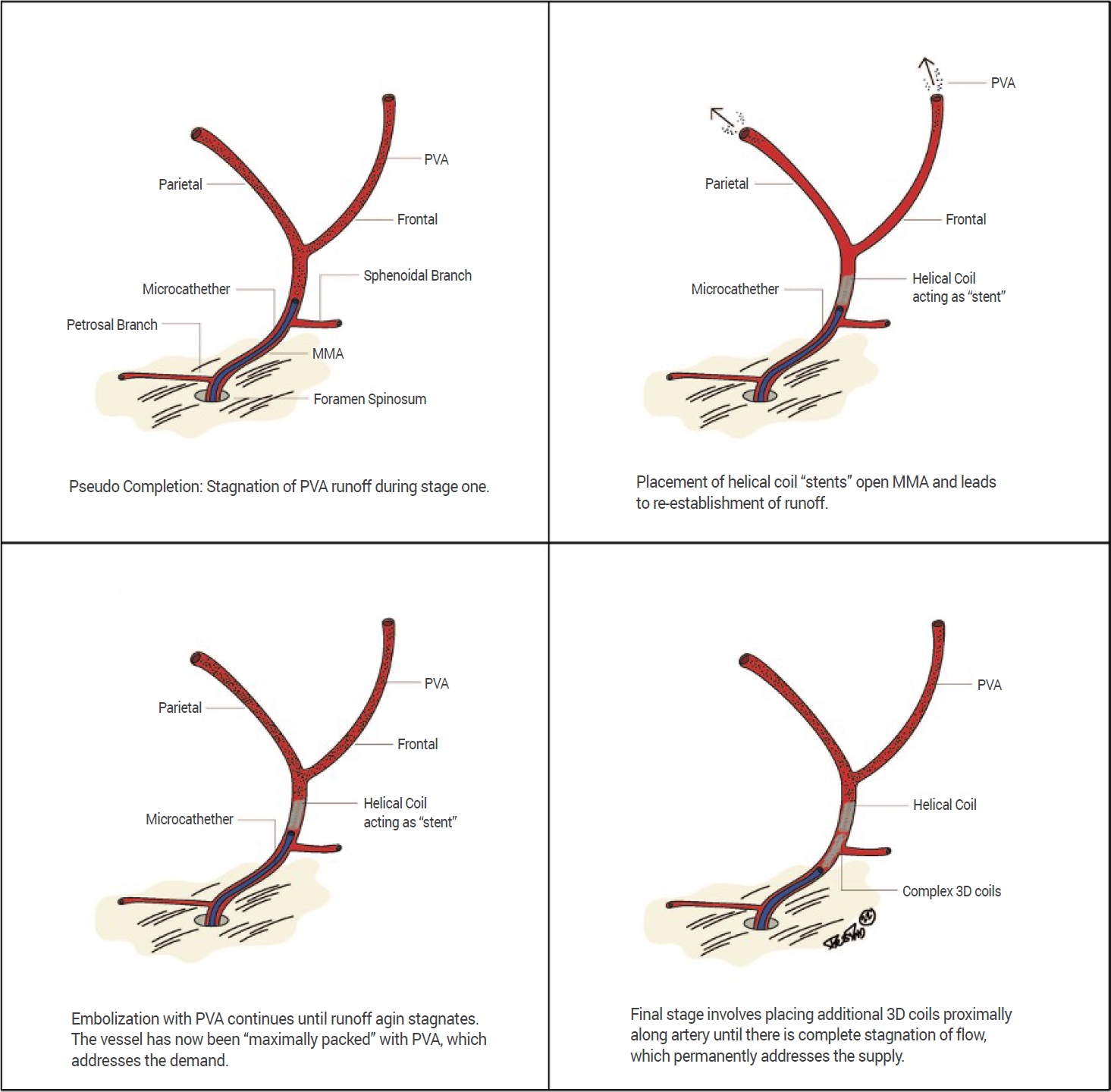
Fig. 2.
Sequential embolization of right middle meningeal artery (MMA). Superselective angiography of the intact right MMA, lateral view (A). Demonstration of flow stasis in right MMA after initial embolisate deployment (B). Restoration of flow through right MMA after deployment of helical coils (C). Further and complete embolization of right MMA after helical coil deployment (D).
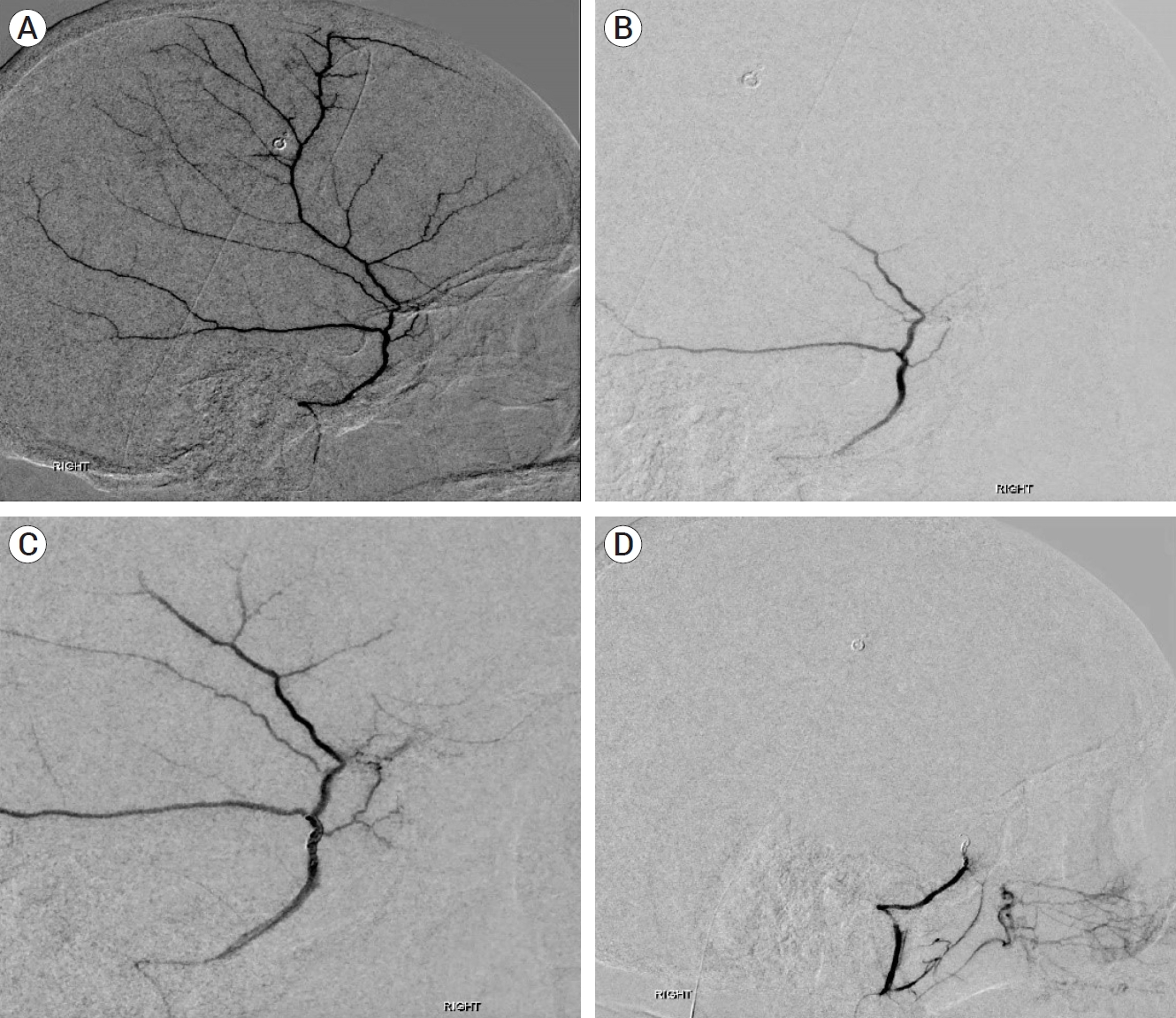
Fig. 3.
Chronic subdural hematoma (cSDH) progression. Axial CT showing bilateral cSDH (arrows) prior to MMA embolization (A). Axial CT scan at 4 months follow-up showing comlete resolution of bilateral cSDH after MMA embolization (B). CT, computed tomography; MMA, middle meningeal artery
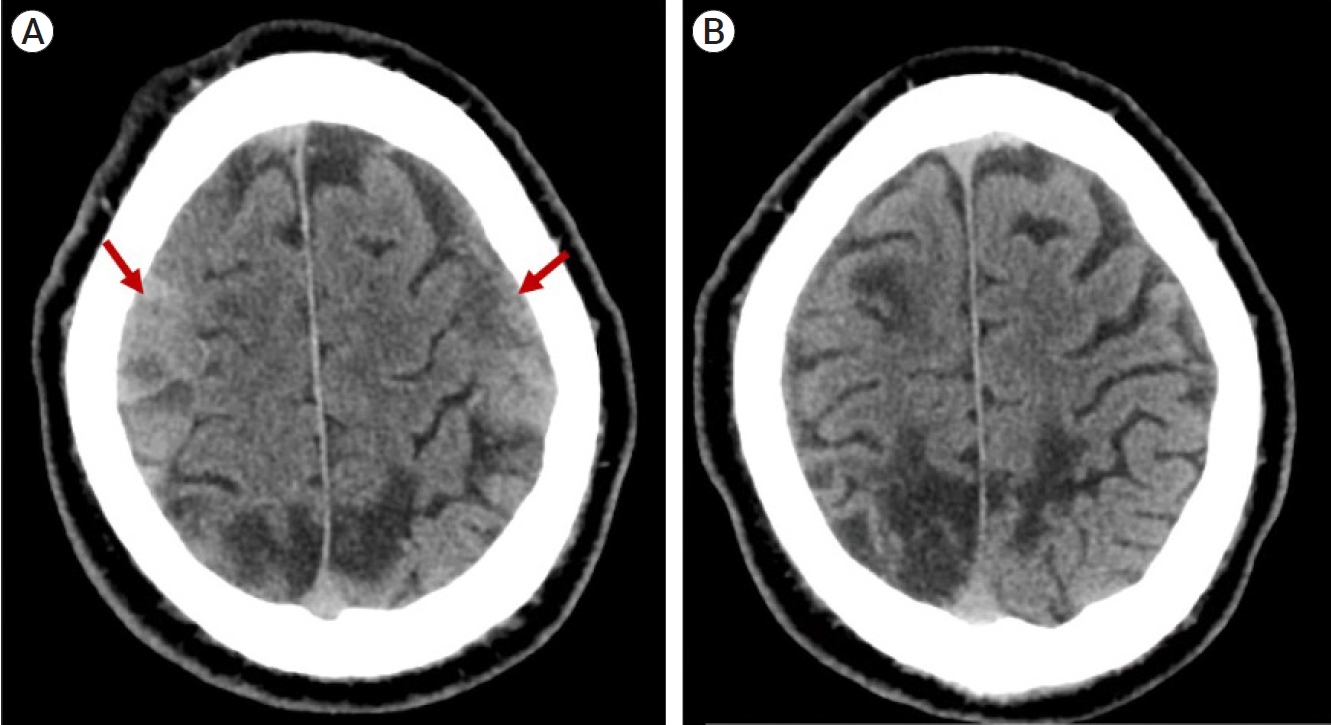
Table 1.
Demographics and baseline parameters
| All patients n=8 (%) | |
|---|---|
| Age (years) | |
| Median (Min, Max) | 80.5 (66, 86) |
| Gender | |
| Female | 2 (25%) |
| Male | 6 (75%) |
| SDH | |
| Unilateral | 3 (38%) |
| Bilateral | 5 (63%) |
| Craniotomy | |
| Before MMA | 1 (12%) |
| After MMA | 0 |
| None | 7 (88%) |
| 3 month follow up | |
| Improvement (reduction in SDH size) | 6 (100%)** |
| No improvement (SDH size increase) | 0 |
| Procedural Complications | |
| Neurologic deficit | 0 |
| IV access point hemorrhage | 0 |
| None | 8 (100%) |
Table 2.
Comparison between prior studies and the current work. Success was defined as stability or reduction in cSDH size. Failure was defined as cSDH expansion or need for surgical intervention.
| Author | Year | # Cases | Method | Success rate |
|---|---|---|---|---|
| Kim [16] | 2017 | 20 | PVA 150-250 microns | 96.2% |
| Ban et al. [2] | 2017 | 72 | PVA 150-250 microns | 98.6% |
| Link et al. [19] | 2018 | 60 | PVA 150-250 microns | 92.0% |
| Ironside et al. [12] | 2021 | 714 | Varied (meta-analysis) | 95.2% |
| Wali et al. | 2022 | 8 | PVA 150-250 microns plus helical coils | 100% |
REFERENCES
1. Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015 Nov;123(5):1209-15.



2. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018 Mar;286(3):992-9.


3. Catapano JS, Ducruet AF, Nguyen CL, Baranoski JF, Cole TS, Majmundar N, et al. Middle meningeal artery embolization for chronic subdural hematoma: an institutional technical analysis. J Neurointerv Surg. 2021 Jul;13(7):657-60.


4. Chen JW, Xu JC, Malkasian D, Perez-Rosendahl MA, Tran DK. The mini-craniotomy for cSDH revisited: new perspectives. Front Neurol. 2021 May;12:660885.



5. Clinical Trials Database; U.S. National Library of Medicine at the National Institutes of Health. Middle meningeal artery embolization AND chronic subdural hematoma. https://clinicaltrials.gov/ct2/results?term=middle+meningeal+artery+-embolization&cond=chronic+subdural+hematoma&age_v=&gndr=&type=Intr&rslt=&Search=Apply (Accessed 09/25/2021).
6. Cofano F, Pesce A, Vercelli G, Mammi M, Massara A, Minardi M, et al. Risk of recurrence of chronic subdural hematomas after surgery: a multicenter observational cohort study. Front Neurol. 2020 Nov;11:560269.



7. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017 May;14(1):108.




8. Gilligan J, Gologorsky Y. Middle meningeal artery embolization for chronic subdural hematoma: current state and future directions. World Neurosurg. 2020 Jul;139:622-3.


9. Gomez-Paz S, Akamatsu Y, Salem MM, Enriquez-Marulanda A, Robinson TM, Ogilvy CS, et al. Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift. Interv Neuroradiol. 2021 Aug;27(4):571-6.



10. Hashimoto T, Ohashi T, Watanabe D, Koyama S, Namatame H, Izawa H, et al. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2013 Aug;4:104.



11. Holl DC, Volovici V, Dirven CMF, Peul WC, van Kooten F, Jellema K, et al. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World Neurosurg. 2018 Aug;116:402-11.


12. Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP, et al. Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J Neurointerv Surg. 2021 Oct;13(10):951-7.


13. Jung YG, Jung NY, Kim E. Independent predictors for recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2015 Apr;57(4):266-70.



14. Kan P, Maragkos GA, Srivatsan A, Srinivasan V, Johnson J, Burkhardt J, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021 Jan;88(2):268-77.

15. Khorasanizadeh M, Salih M, Harris D, Ogilvy CS. Technique to “trap” a segment of the middle meningeal artery for embolization of a carotid cavernous fistula: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2021 Oct 13 21(5):e444.



16. Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017 May;101:520-7.


17. Leroy HA, Aboukaïs R, Reyns N, Bourgeois P, Labreuche J, Duhamel A, et al. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J Clin Neurosci. 2015 Dec;22(12):1895-900.


18. Link TW, Boddu S, Marcus J, Rapoport BI, Lavi E, Knopman J. Middle meningeal artery embolization as treatment for chronic subdural hematoma: a case series. Oper Neurosurg (Hagerstown). 2018 May;14(5):556-62.



19. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. 2019 Dec;85(6):801-7.



20. Link TW, Rapoport BI, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: endovascular technique and radiographic findings. Interv Neuroradiol. 2018 Aug;24(4):455-62.




21. Matsumoto H, Hanayama H, Okada T, Sakurai Y, Minami H, Masuda A, et al. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci. 2018 May;49:40-7.


22. Mishra A, Kumar A, Mathur A, Kumar V, Sreen A. Coil assisted glue embolization to improve safety and accuracy in endovascular management of Vein of Galen patients. Clin Neurol Neurosurg. 2021 Apr;205:106652.


23. Moshayedi P, Liebeskind DS. Middle meningeal artery embolization in chronic subdural hematoma: implications of pathophysiology in trial design. Front Neurol. 2020 Aug;11:923.



24. Ng S, Derraz I, Boetto J, Dargazanli C, Poulen G, Gascou G, et al. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: a pilot study assessing hematoma volume resorption. J Neurointerv Surg. 2020 Jul;12(7):695-9.


25. Nia AM, Srinivasan VM, Lall RR, Kan P. Middle meningeal artery embolization for chronic subdural hematoma: a national database study of 191 patients in the united states. World Neurosurg. 2021 Sep;153:e300-7.



26. Peng D, Zhu Y. External drains versus no drains after burrhole evacuation for the treatment of chronic subdural haematoma in adults. Cochrane Database Syst Rev. 2016 Aug;2016(8):CD011402.



27. Rutledge C, Baranoski JF, Catapano JS, Jadhav AP, Albuquerque FC, Ducruet AF. Resolution of an enlarging subdural haematoma after contralateral middle meningeal artery embolisation. BMJ Case Rep. 2021 Apr;14(4):e017530.



28. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burrhole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009 Sep;374(9695):1067-73.


29. Shotar E, Meyblum L, Premat K, Lenck S, Degos V, Grand T, et al. Middle meningeal artery embolization reduces the post-operative recurrence rate of at-risk chronic subdural hematoma. J Neurointerv Surg. 2020 Dec;12(12):1209-13.


30. Sjåvik K, Bartek J Jr, Sagberg LM, Henriksen ML, Gulati S, Ståhl FL, et al. Assessment of drainage techniques for evacuation of chronic subdural hematoma: a consecutive population-based comparative cohort study. J Neurosurg. 2017 Jun;133(4):1113-9.

31. Sousa EB, Brandão LFS, Tavares CB, Borges IBC, Neto NGF, Kessler IM. Epidemiological characteristics of 778 patients who underwent surgical drainage of chronic subdural hematomas in Brasília, Brazil. BMC Surg. 2013 Mar;13:5.




32. Tamura R, Sato M, Yoshida K, Toda M. History and current progress of chronic subdural hematoma. J Neurol Sci. 2021 Oct 15 429:118066.


33. Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008 Dec;63(6):1125-9; discussion 1129.

34. Xu C, Zhu Y; External drains versus no, Yang X, Wei M, et al. Randomized controlled study on the curative effects of twist-drill craniotomy and burr-hole craniotomy in the treatment of chronic subdural hematoma. Exp Ther Med. 2018 Aug;16(2):959-65.



- TOOLS
-
METRICS

-
- 3 Crossref
- 0 Scopus
- 3,032 View
- 60 Download
- ORCID iDs
-
David R. Santiago-Dieppa

https://orcid.org/0000-0003-4429-0030 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



