Association between ischemic stroke and pyogenic spondylitis in Korea: Nationwide longitudinal cohort study
Article information
Abstract
Objective
The purpose of this nationwide age- and sex- matched longitudinal study was to determine the pyogenic spondylitis (PS) increases the incidence of ischemic stroke (IS) in Korea.
Methods
From the National Health Insurance Service (NHIS), we collected the patient data for the period from January 1, 2004 to December 31, 2015. PS was classified according to the International Classification of Disease codes M46.2-M46.8, M49.2, and M49.3. By using a 1:5 age- and sex- stratified matching, a total of 628 patients and 3140 control subjects were included in the study. The IS incidence rates in PS and control group was calculated by using the Kaplan-Meier method. The outcome of hazard ratio of IS was estimated by Cox proportional hazards regression analyses. This study did not exclude PS as a result of postoperative complications.
Results
According to the study, 51 patients (8.12%) in the PS group and 201 patients (6.4%) in the control group experienced IS. The adjusted hazard ratio of IS in the PS group was 3.419 (95% CI: 2.473-4.729) after adjusting individual medical condition and demographics. Following the results of subgroup analysis, the risk ratio of IS was greater in most of the subgroup categories (male, female, age <65, age >65, non-diabetic, hypertensive, non-hypertensive, dyslipidemic and non-dyslipidemic subgroup). However, the risk of IS did not differ significantly in diabetic subgroup (95% CI: 0.953-4.360).
Conclusions
The risk rate of IS increased in patient with pyogenic spondylitis.
INTRODUCTION
Pyogenic spondylitis (PS) is rare disease that covers a broad range of clinical area including pyogenic spondylodiscitis, septic discitis, vertebral osteomyelitis, and epidural abscess [3,13,15,16,26]. However, the incidence of PS is increasing due to the increase in the elderly and immunocompromised patients [3,13,26]. Several studies have been conducted on the relationship between PS and infection [3,15,16,26]. It is usually caused by hematogenous spread of bacteria [3,14,26]. However, there were no studies and information on the direct relationship between the increase in ischemic stroke (IS) by PS. This nationwide longitudinal study was conducted to clarify whether the infectious disease, PS, increases the incidence of IS. This study did not exclude PS as a result of postoperative complications.
MATERIALS AND METHODS
Data source
In Korea, single payer health insurance system is managed by National Health Insurance System (NHIS) [16,17,23]. Regular and part-time workers over the age of 40 are recommended to undergo regular health checkups every 1 or 2 years. The collected information (e.g., demographic profile, health insurance claims, national health examination results and death certificates) is saved in National Health Information Database (NHID). For this study, data from 2004 to 2015 were provided from NHIS-HEALS cohort. We were able to access the data legally for research purposes with the approval of the institutional review boards (IRB No. 2020-01-011).
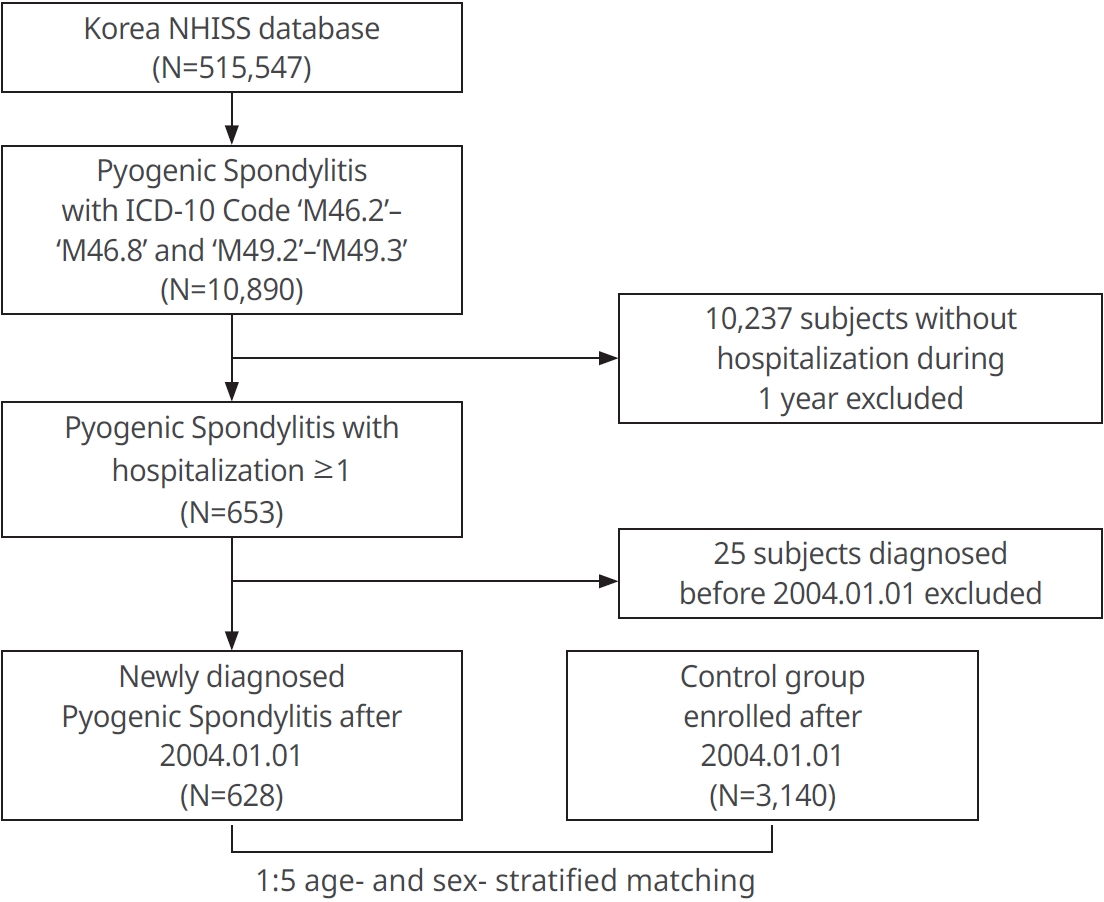
Establishment of the study cohort
The group diagnosed with PS according to the ICD-10 code (M46.2-M46.8, M49.2-M49.3) was included in the PS group. Only 10,890 PS subjects were selected out of a total of 15,547 patients in the NHISS database. By excluding those diagnosed with PS before Jan. 1, 2004 and 10,237 patients who had never been hospitalized, a group of 628 patients newly diagnosed with PS after Jan. 1, 2004 could be derived and were monitored until Dec. 31, 2015. By using the greedy matching algorithm of the ‘Match IT’ R package, 3,140 people were selected as a control group in 1:5 age- and sex- stratified matching (without replacement) [18,19]. The patient groups included in this study were followed from the first diagnosis of IS until death or the end of the study (Fig. 1). Among the PS and control group, IS patients were selected by using the following criteria: ICD-10 codes (I63 or I64), IS confirmed by brain CT or MRI scan and hospitalization ≥1 day [17,18]. The risk of IS was evaluated by adjusting for other factors such as age, sex and comorbidities (hypertension, diabetes mellitus, and dyslipidemia), and values could be derived accordingly.
Statistical analysis
The chi-squared tests and student’s t-test were used to evaluate the demographic diversity and characteristics between the PS group and the control group. The Kaplan-Meier method was able to calculate the viability without IS in each group. The differences in diseasefree survival among the two groups were assessed by using the Wilcoxon’s log rank test. Multivariate analysis of the Cox proportional hazard regression model was performed to determine the effect of PS on IS. Two Cox proportional hazard regression models were designed and analyzed through R software (version 3.3.3, The R Foundation for Statistical Computing, Vienna, Austria). For Model 1, age and sex were adjusted, and for Model 2, age, sex, and underlying disease were adjusted. To adjust covariates, we conducted subgroup analyses.
RESULTS
Characteristics of the PS and control group
For the PS group, 628 patients newly diagnosed with PS were selected and 3140 patients were selected for the control group. Of these, 323 patients (51.43%) were male and 199 patients (48.57%) were female. The mean age of the patients was 59.09±9.35 years. In the presence of diabetes, there was difference in prevalence between the two groups (P<0.01) (Table 1).
IS in the PS and control group
The likelihood of the occurrence of IS was higher in the PS group than that of IS in the control group. Through the Kaplan-Meier curves, the cumulative incidence of IS was higher in the PS group (Fig. 2). Multivariate analysis of Cox proportional hazards regression Model 1 showed that the IS hazard ratio for the PS group was 3.518 compared to the control group (95% CI: 2.546-4.801, Table 2). In the Model 2, the IS hazard ratio was 3.419 compared to the control group (95% CI: 2.473-4.729, Table 2).
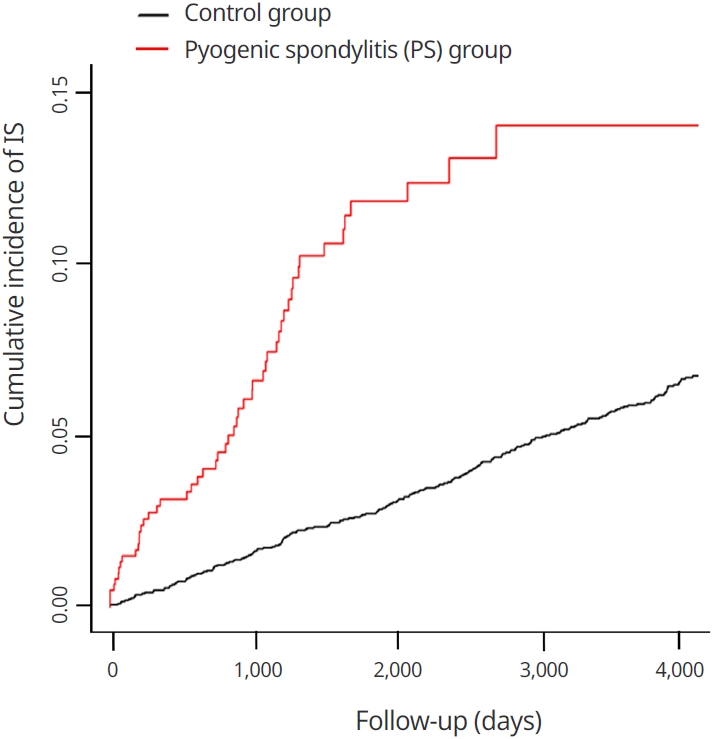
The Kaplan-Meier curves with cumulative incidence of ischemic stroke in the pyogenic spondylitis and control groups.
Subgroup analysis of IS incidence rate
The IS incidence rate was different between PS and control group in both of male (95% CI: 1.953-4.935, Table 3) and female subgroup (95% CI: 2.517-6.207, Table 3). The incidence of IS was significantly different between PS and control group in both of age <65 (95% CI: 3.173-7.731, Table 3) and age >65 subgroup (95% CI: 1.473-3.852, Table 3). The IS incidence rate was different in both of non-hypertensive (95% CI: 2.045-5.183, Table 3) and hypertensive subgroup (95% CI: 2.373-5.826, Table 3). Furthermore, the incidence of IS was significantly different in non-dyslipidemic (95% CI: 2.509-4.960, Table 3), dyslipidemic (95% CI: 1.165-8.768, Table 3) and non-diabetic subgroup (95% CI: 2.652-5.414, Table 3). However, there was no significant difference between PS and control group in diabetic subgroup (95% CI: 0.953-4.360, Table 3).
DISCUSSION
According to our study, there was 3.518 fold increased incidence of IS after adjusting sex and age and 3.419 fold increased incidence of IS after adjusting sex, age, and underlying disease (diabetic, dyslipidemia, hypertension). The study results showed that the incidence of IS was higher in the PS group in male, female, under 65 years of age, over 65 years of age, non-diabetic, non-dyslipidemic, dyslipidemic, non-hypertensive and hypertensive.
PS is an uncommon spinal infection that can give patients a serious and life-threatening condition [3,15,16,26]. The most common organism of PS is S. aureus [3,13,15,16,26]. Research on PS reports diabetes mellitus (DM), long-term steroid therapy, substance abuse, malnutrition, malignancy, liver cirrhosis and HIV as risk factors [4,12,14,15,26]. Currently, there is a lack of research and information on the direct relationship between PS and IS. However, several studies have been conducted on the link between infection and IS. Acute and chronic infections have been reported to accelerate the progression of atherosclerosis, leading to acute ischemic stroke [7,11,12,17,18,29]. Grau et al. [10] reported bacterial infections significantly increased the risk for cerebrovascular ischemia (95% CI, 2.0 to 16.9; P<.002). In addition, it has been reported that C-reactive protein (CRP) can be used as a strong indicator in relation to vascular disease [5,9]. Increased lipoprotein and plasminogen activator inhibitor-1 can be used as biomarkers for the progression of intracranial atherosclerotic disease [1,2,6,10,12,17,20,22,25,29,30]. Ross et al. [25] reported that inflammation is associated with an increase in fibrinogen and CRP, both of which are associated with an early sign of atherosclerosis. In addition to that, interleukin-6 (IL-6) was reported as a major inflammatory cytokine associated with a predictive marker of stroke risk [8,9,20,21,24]. Although infection increases the risk of IS, it has been reported that IS increases the risk of infection by inducing immunosuppression [7,12,27,28]. Several studies have shown that infection and IS are closely related [4,7,8,10,12,21,24,27-29].
Interpretation of the graph (Fig. 2) becomes possible through these inflammatory factors. It can be seen that the slope of the graph is steep in the early stage of diagnosis, because IS occurred in many patients due to atherosclerotic changes caused by inflammatory factors. A delayed onset of IS was seen in the remaining patients with intracranial atherosclerotic changes. Therefore, it can be seen that the slope of the graph becomes smoother over time. Near the end of the follow-up period, it shows a flat slope which indicates no accumulation of IS after a long period of time. This is because the IS had already occurred at the beginning and middle of the follow-up period.
Since PS is a disease caused by infection of the spine, it can be expected that the risk of IS may be increased. However, further studies are needed to elucidate the direct causes and mechanisms.
There are several limitations in our study. First, this study was conducted by classifying gender, age, and underlying disease in the NHIS database, and did not include individual lifestyles (Alcohol, tobacco, regular diet and exercise, etc.) [18,19] and biomarkers (CRP, IL-6, fibrinogen, lipoprotein, and plasminogen activator inhibitor-1 etc.) [1,2,6,8,10,12,17,20-22,24,25,29,30]. Second, information on the clinical severity of individual PS patients was not included in the data. Further studies may be required due to the difficulties in randomization to various factors not included in the data.
While there may be shortcomings in several areas, this is the largest nationwide longitudinal study showing an increased risk of IS in patients with PS.
CONCLUSIONS
Through this nationwide longitudinal study, it was found that the risk of IS was increased in PS patients.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A1A01072258). This work was also supported by a grant of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2020R1F1A1069875).
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.