Initial experience with Scepter Mini dual lumen balloon for embolization of cerebrovascular diseases
Article information
Abstract
Objective
Endovascular treatment of cerebrovascular diseases is often challenging due to small caliber, tortuous distal vessels. Several devices and techniques have evolved to overcome these challenges. Recently, a low profile dual lumen microballoon catheter, specifically designed for distal navigation is employed for neurovascular procedures. Due to its recent advent, scarce data is available on clinical utility and safety of Scepter Mini. The aim of this case series is to report our initial experience with Scepter Mini in the management of various cerebrovascular diseases.
Methods
All interventional neurovascular cases performed using Scepter Mini between January 2020 till April 2021 were included. Data regarding patient demographics, procedural details and complications was retrospectively collected from patient’s electronic medical record and procedure reports.
Results
Total twelve embolization procedures were performed in eleven patients, including six brain arteriovenous malformation, two dural arteriovenous fistula, one vein of Galen malformation and three hyper-vascular glomus tumor embolizations. All procedures were successfully performed with adequate penetration of the embolic agent. Complete embolization was performed in six procedures, while intended partial embolization was performed in the rest of procedures. Scepter Mini was solely used in ten procedures, however in the other two embolization procedures it was used as an additional conjunct tool to complete the intended embolization. No balloon related complication was observed in any procedure.
Conclusions
Scepter Mini dual lumen microballoon catheter is safe and feasible for delivery of liquid embolic agents for cerebrovascular embolization procedures.
INTRODUCTION
Endovascular embolization is a well-established treatment option for various cerebrovascular diseases. Several agents have been used for embolization, including coils, N-butyl cyanoacrylate (NBCA), embolic particles and more recently liquid embolic agents. Irrespective of the embolization material, several important factors contribute towards the successful endovascular treatment of these diseases [8,12,17]. One such factor is gaining distal access, as close as possible to the target pathology. Often tortuous anatomy and small caliber of distal vasculature make this challenging [10]. To overcome this factor, recent device innovations have led to development of supple, smaller caliber microcatheters to allow easy navigability into distal vasculature [7]. Another important limiting factor for liquid agent embolization procedures is the reflux of embolization material into the parent feeding vessel. This can lead to inadequate distal penetration and pre-mature cessation of embolization, which jeopardize adequate treatment [8,17]. Several techniques have been described to minimize this factor [1,4]. Often these techniques require placement of two simultaneous microcatheters in the feeding vessel, which may not be feasible in small caliber, tortuous vessels [4]. In such scenarios, detachable tip microcatheter is another option, which is specifically designed to overcome the limiting factor of reflux during embolization [5,11]. Variable detachable tip lengths are available which allow operators to have controlled reflux up to a certain point. However, in some instances, the length of feeding vessel is smaller than the available detachable tip length of microcatheter or a branch supplying normal brain parenchyma may be too close to the target, limiting the room for reflux. To overcome these issues, more recently, dual lumen balloon microcatheters have been employed to limit the reflux of liquid embolic agents and better distal penetration during embolization procedures [2,6]. The main limitation of these previously available dual lumen balloon microcatheters is large caliber (0.017 inches), often not feasible for distal navigation into tortuous, small vessels [8]. A recently available, lower profile dual lumen microballoon catheter is specifically designed for navigation into distal, smaller arteries, which can help to improve endovascular embolization procedures [16]. Due to its recent commerical availability, scarce clinical data is available on utility and safety of Scepter Mini balloon. The purpose of this case series is to report our initial experience regarding the use of Scepter Mini balloon catheter for various cerebrospinal vascular embolization procedures.
MATERIALS AND METHODS
Retrospective review was performed for all interventional neurovascular procedures performed using Scepter Mini between January 2020 till July 2021. Data regarding patient demographics, procedural details and complications were recorded from patient’s electronic medical record and procedure reports.
Technical details of embolization procedures
All embolization procedures were performed under general anesthesia. Two embolization procedures were performed via distal radial approach, one via ulnar approach, while others were performed via trans-femoral approach. Pre-embolization angiogram was performed in all procedures to establish the target vessels for embolization. Scepter Mini (MicroVention Inc., Aliso Viejo, CA, USA) was prepared in all instances according to the manufacturer’s information. The catheter related lumen was flushed with heparinized saline, while the balloon related lumen was filled with slow injection of pure iodinated contrast agent, Visipaque 320 (Iodixanol, GE HealthCare Technologies Inc., Chicago, IL, USA) using the 0.2 cc syringe pre-packaged with the balloon. The balloon is held in upright position to visually purge the air from the balloon lumen. Once the balloon is inflated with no residual air, it is subsequently deflated completely under saline with negative suction. In all instances, the balloon catheter was navigated over 0.008″ Asahi Chikai Neurovascular wire (Asahi Intecc Inc., Tustin, CA, USA) to the target vessel under roadmap guidance. In four procedures with intracranial target vessel, a tri-axial system using an intermediate catheter was used to support the balloon navigation. In all other procedures, with extra-cranial target vessel, a bi-axial system with direct advancement of balloon through the guide catheter was used. Super-selective angiogram was performed in all instances through the Scepter Mini to assess the adequate positioning before embolization. Once satisfactory position was confirmed, the balloon was gently inflated under roadmap guidance using pure iodinated contrast agent. The balloon inflation was titrated according to the size of target vessel, which was visually assessed on live roadmap images. After balloon inflation, the balloon lumen was locked using three way stop cock to prevent auto-deflation. The catheter lumen was then primed with Dimethyl sulfoxide (DMSO) and subsequently the liquid embolic agent was injected slowly under live fluoroscopic and roadmap guidance. The injection was stopped as and when required to prevent non-target embolization. In instances of embolic agent reflux proximal to the inflated balloon, the balloon was gently re-inflated to prevent further reflux. Interim control angiography runs were performed through the base catheter to assess degree of embolization. Once target embolization was achieved, the balloon was deflated and removed under live fluoroscopy. Final standard angiogram was performed before the catheters and sheath were removed. All patients undergoing embolization were admitted to Neuro Intensive Care Unit for post procedure care.
RESULTS
Scepter Mini was used for twelve embolization procedures in eleven patients. Seven procedures required intra-cranial target vessel embolization; six brain arteriovenous malformations, one vein of galen malformation, while five other procedures required extra-cranial target vessel embolization; two dural arterio-venous fistulas and three hypervascular glomus tumors (Table 1). Target vessel was successfully engaged in all procedures with easy navigability of Scepter Mini balloon to the intended location. Pre-planned degree of embolization was achieved in all procedures. Complete embolization was performed in six procedures, including two brain arteriovenous malformations (AVMs), two dural AVFs and two glomus tumors, while partial embolization (>50%) was performed in rest of the procedures (Fig. 1 and 2). In four instances during different embolization procedures, reflux of embolic agent was noted proximal to the inflated balloon, which was managed with gentle re-inflation of the balloon. None of the proximal reflux was significant, requiring repositioning of the balloon. There was no instance of balloon glued within the target vessel.
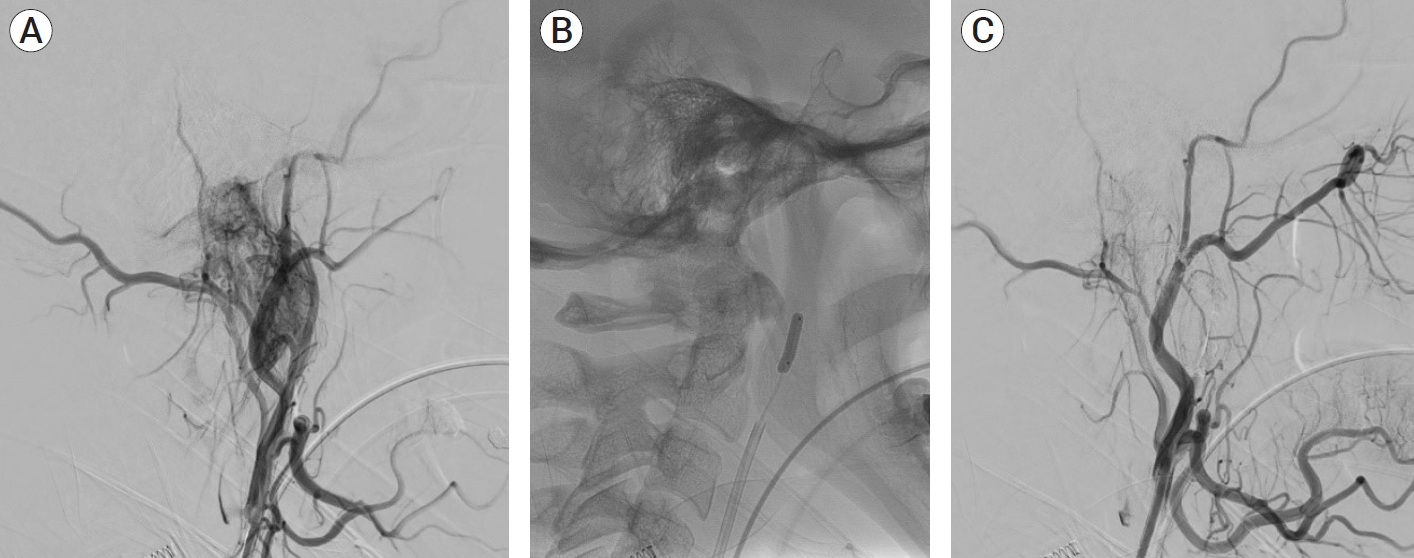
(A) Initial angiogram of the right external carotid artery demonstrates hypervascular glomus tumor with arterial feeders arising from ascending pharyngeal artery. (B) Inflated Scepter Mini balloon within the ascending pharyngeal artery. (C) Post embolization angiogram shows complete embolization of the tumor
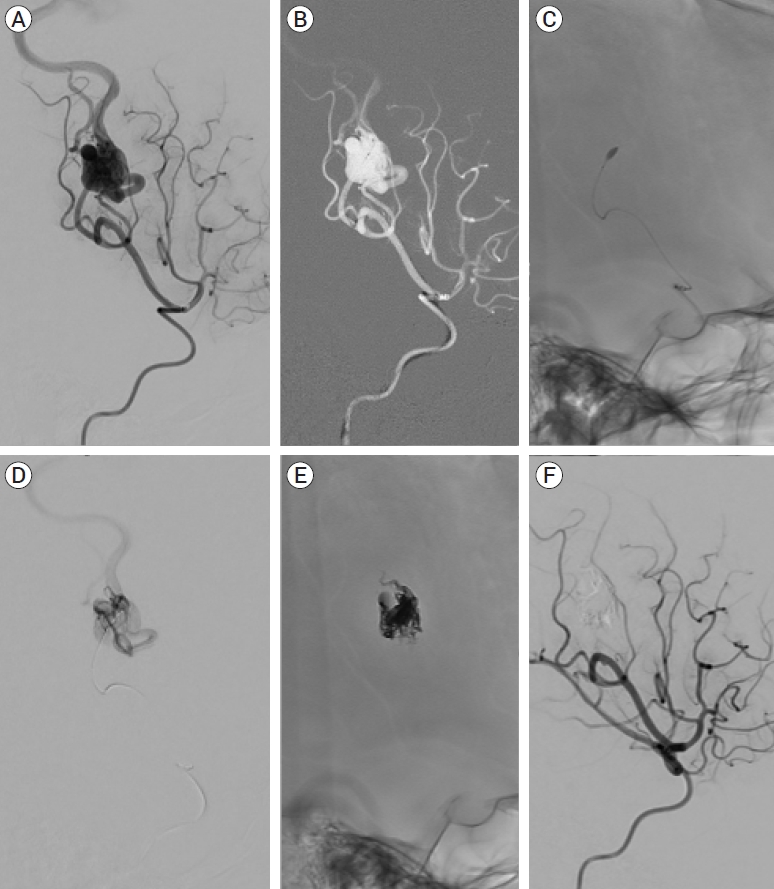
(A) Angiogram of the right middle cerebral artery demonstrates a posterior parietal AVM. (B) Roadmap image shows navigation of Scepter Mini balloon catheter into the arterial feeder. (C) Inflated Scepter Mini balloon catheter into the arterial feeder. (D) Selective angiogram through the Scepter Mini balloon outlining the compact AVM nidus. (E) Onyx cast matching the AVM nidus. (F) Post embolization angiogram demonstrates complete obliteration of the AVM with no residual filling of the nidus. AVM, arteriovenous malformation
Scepter Mini was used as the sole embolization catheter in ten out of twelve (83%) embolization procedures without use of other adjunct embolization devices. In two embolization procedures, initial partial embolization was performed with detachable-tip microcatheters and Scepter Mini was employed at later embolization session to complete the intended procedure. Liquid embolic agents were used in all embolization procedures, including Onyx-18 in eleven and PHIL-25% in one procedure. No balloon or procedure related complications were observed in any of the patients.
DISCUSSION
Emerging clinical and technical needs have led to rapid innovation of medical devices. One such recent advancement in neurointerventional tools is Scepter Mini, a low-profile dual lumen microballoon catheter. It bears a smaller profile at the distal tip (1.6 French) as compared to its predecessors, Scepter C and Scepter XC (MicroVention Inc., Aliso Viejo, CA, USA) balloon catheters (2.6 French). This low profile characteristic make the distal tip more supple, which helps in distal navigation of the balloon catheter across the tortuous cerebral vasculature [9,15]. Besides the lower profile, another advantage of these balloon microcatheter is dual lumen design. Previously described technique for better penetration and limiting liquid embolic agent reflux required two simultaneous catheters, one needed for proximal control and the other for distal embolization [3]. However, with the advent of dual lumen balloon catheters, both proximal control and distal embolization can be achieved with a single catheter [2]. The catheter lumen can be used independently to inject contrast or embolic agents while the separate balloon lumen is used to inflate/deflate the balloon. Proximal balloon inflation assists in several ways during embolization procedures. It aids to control the distal blood flow which allows better penetration of liquid embolic agents and also prevent early distal embolization. More importantly, proximal balloon inflation limits the reflux of embolic agent by providing a physical barrier in the parent artery. This control of proximal reflux entails faster procedures with better distal penetration, lower chances of non-target embolization and lesser risk of catheter entrapment [13]. A pre-clinical study comparing Scepter Mini with the standard microcatheter demonstrated significantly shorter procedure time with increased embolization extent and decrease reflux events [16]. Since it’s clinical use is novice, scarce data is available to establish its safety and technical success. Recently, Vollherbst et al. published the first multicenter clinical safety for this device [15]. They reported easy or very easy navigability in most of their cases. No device related complication was reported in their experience.
Despite being lower profile, Scepter Mini is not devoid of the inherent risks associated with balloon assisted procedures. Vessel perforation, a well-known complication is associated with over inflation of balloon beyond the vessel caliber [8,14]. Since Scepter Mini can track to distal vessels in comparison to the other dual lumen balloons, the potential risk of vessel perforation is even higher due to smaller vessel sizes. Although, we did not encounter this potential complication, the operators in general should be extra cautious, while inflating the Scepter Mini balloon. In our experience, the balloon should be navigated close to the nidus as safely possible and gentle inflation should be performed under roadmap guidance to guide the size of inflated balloon. Vollherbst et al. suggested to be careful about stopcock as the balloon lumen has small volume and any inadvertent increase/push of small volume can result in overinflation of the balloon [15].
While the low profile design has advantages of easy navigability, it does harbor an inherent disadvantage of catheter stability. Since the distal end of Scepter Mini is supple, it can jeopradize stability resulting in unstable positioning of the balloon catheter within target vessel. We observed this phenomenon in three instances with minimal proximal migration of the balloon during injection of embolic agent. This was overcome with gentle over-inflation of the balloon to anchor it well within the target vessel. Vollherbst et al. reported technical failure in 4/26 procedures due to similar unstability of Scepter Mini within the target vessel [15]. This instability was mostly observed in larger diameter vessels, mostly more than 2 mm. Since the diameter of inflated Scepter Mini balloon is 2.2 mm, it may not be feasible for use in larger caliber vessels.
Minimal reflux proximal to the inflated balloon was observed in 3/11 procedures which did not result in premature cessation of embolization procedure or resulting in catheter stuck in the vessel. In all these instances the balloon was carefully inflated with more volume to provide better seal against the vessel wall. In our observation if the stopcock is left open for the balloon lumen, the balloon can deflate gradually, providing a roam for proximal reflux. Once the balloon is inflated, caution should be paid to close the stopcock towards the balloon lumen to prevent inadvertent deflation of balloon at any time during the procedure.
We recognize several limitations in our case series including the retrospective nature and small number of patients. We performed intracranial as well as extra-cranial embolization procedures, however we did not utilize Scepter Mini for spinal embolization procedures, navigation support, balloon assisted coiling or other balloon-assisted neurovascular procedures. The size of target vessel, total amount of liquid embolic agent and embolization time was not available for all procedures.
CONCLUSIONS
Scepter Mini balloon catheter is safe for various cranial and head and neck embolization procedures with better distal navigation capability. It’s true potential beyond embolization procedures need to be explored in large prospective studies.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.