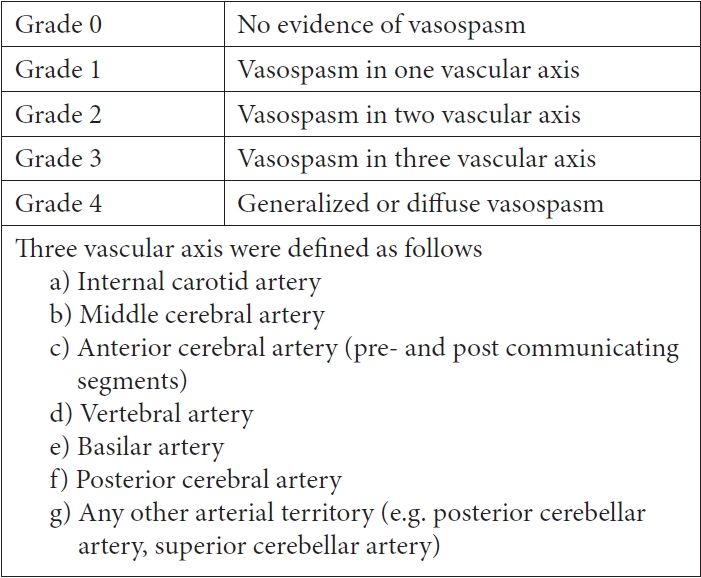
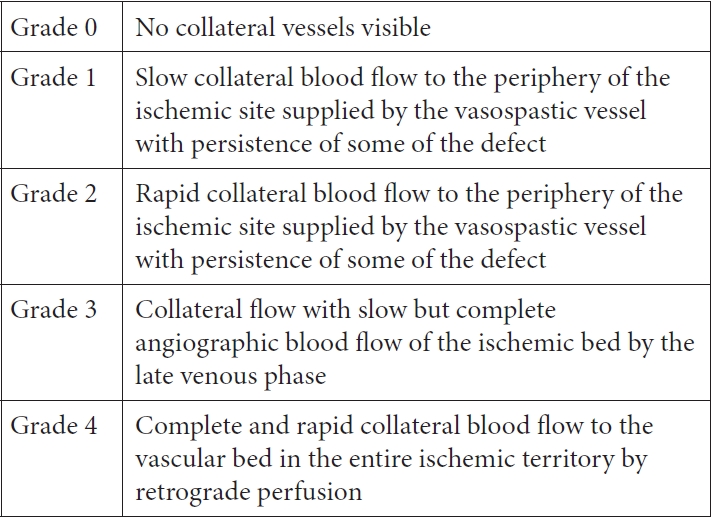
The impact of collateral status on cerebral vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage
Article information
Abstract
Objective
Cerebral collateral circulation may affect subarachnoid hemorrhage (SAH) induced cerebral vasospasm and delayed cerebral ischemia. In this study our aim was to investigate the relationship between collateral status, vasospasm and delayed cerebral ischemia (DCI) in both aneurysmal and nonaneurysmal SAH.
Methods
Patients diagnosed as SAH with and without aneurysm were included and their data investigated retrospectively. After the patients diagnosed as SAH according to cerebral computed tomography (CT)/magnetic resonance imaging (MRI), they underwent cerebral angiography to check for cerebral aneurysm. The diagnosis of DCI was made according to the neurological examination and control CT/MRI. All the patients had their control cerebral angiography on days 7 to 10 in order to assess vasospasm and also collateral circulation. The American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) Collateral Flow Grading System was modified to measure collateral circulation.
Results
A total of 59 patients data were analyzed. Patients with aneurysmal SAH had higher Fisher scores and DCI was more common. Although there was no statistically significant difference between the patients with and without DCI in terms of demographics and mortality, patients with DCI had worse collateral circulation and more severe vasospasm. These patients had higher Fisher scores and more cerebral aneurysm overall.
Conclusions
According to our data, patients with higher Fisher scores, more severe vasospasm, and poor cerebral collateral circulation may experience DCI more frequently. Additionally aneurysmal SAH had higher Fisher scores and DCI was seen more common. To improve the clinical results for SAH patients, we believe that physicians should be aware of the DCI risk factors.
INTRODUCTION
In spite of many worldwide studies, the pathogenesis and best management of delayed cerebral vasospasm due to subarachnoid hemorrhage (SAH) remains still unclear. Up to 70% of patients have radiographic evidence of vasospasm starting on day 3 after SAH, maximal at 5–14 days and resolves on day 21 [6,15]. The presence of severe hemorrhage seen on admission computed tomography (CT) is strongly associated with higher risk for vasospasm. Delayed cerebral ischemia (DCI) is defined as the development of new focal neurological signs and/or deterioration in level of consciousness, lasting for more than 1 h, or the appearance of new infarctions on CT or magnetic resonance imaging (MRI). The underlying pathophysiology is vasospasm and other causes are excluded [27].
CT angiography and MRI angiography can be also used for diagnosis. One important factor to remember is that not all patients with cerebral vasospasm experience DCI [28]. There should be some other factors involved. Recent studies showed that in acute cerebral ischemia, cerebral collateral circulation is an independent predictor of reperfusion, final infarct size, and clinical outcome; however, its impact on DCI in SAH patients remains uncertain [10,14]. We believe that not only vasospasm but also collateral status is considerable for SAH patients with DCI. In this study we investigated the collateral status and DCI according to angiographic findings in SAH patients with and without cerebral aneurysm retrospectively. We believe that the incompetent collateral circulation may have adverse effect on DCI development which can be taken care by the physicians in such patients.
METHODS
This single-center, retrospective, cohort study included 59 SAH patients who admitted to our Neurosurgery Department between February 2015 and May 2018. The diagnosis of SAH was done according to noninvasive neuroimaging studies including CT, MRI, CT angiography (CTA) and MR angiography (MRA). The Fisher scores were used to analyze the SAH severity and demographic information were all recorded.
Initial cerebral DSA procedure was performed in the angiography suite by an endovascular neurosurgeon within 2-48 hours (mean 6.8±1.2 h). According to the initial cerebral DSA results, the patients were split into two groups; aneurysmal and non aneurysmal SAH.
Patients with a previous history of SAH or presence of vascular pathology including arteriovenous malformation, intracranial vasculitis, cerebral vasoconstriction, mycotic aneurysms and a shunting vascular malformation or under any kind of medication causing vasoconstriction were excluded. Both aneurysmal and nonaneurysmal SAH patients were included in this study. Nonaneurysmal SAH patients were those having no aneurysms in their initial cerebral angiography. These patients had traumatic or perimesencephalic SAH or hemorrhage due to anticoagulant drugs. Clinical management was done according to the American Heart Association and the Neurocritical Care Society SAH guidelines [4]. Aneurysmal SAH patients had either endovascular coiling or surgical clipping as soon as possible. During the follow up period the patients had control CT/MRI and the patients with new focal neurological signs and/or deterioration in level of consciousness, lasting for more than 1 h, or the appearance of new cerebral infarctions on CT/MRI were diagnosed as DCI. The diagnosis of DCI was done according to the neurological examination and control CT/MRI findings. In case of progression in neurological clinic control CT/MRI was immediately performed. Subsequently all patients had a follow-up cerebral DSA by an endovascular specialist within 7-10 days after the initial DSA. Cerebral vasospasm and collateral status were assessed according to the second cerebral DSA findings.
The collateral circulation were assessed according to the findings of second cerebral DSA by using a modification of the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) Collateral Flow Grading System (Figs. 1 and 2) [9] which had been used in DCI in previous studies [1]. Cerebral vasospasm was also determined according to the comparison of first and second DSA findings based on angiographic scale (Fig. 3) [10,19]. Patients with vasospasm had intraarterial vasodilator (calcium channel blocker nimodipine) during procedure and subsequently had medical management of vasospasm consisted of intravenous nimodipine for at least 2 weeks, mean arterial pressure control (>90 mmHg), adequate hydration with central venous pressure monitoring. We administered nimodipine at a dosage of 15 microgram/kg/hour. The infusion is done through central venous catheter with close blood pressure monitoring in intensive care unit.
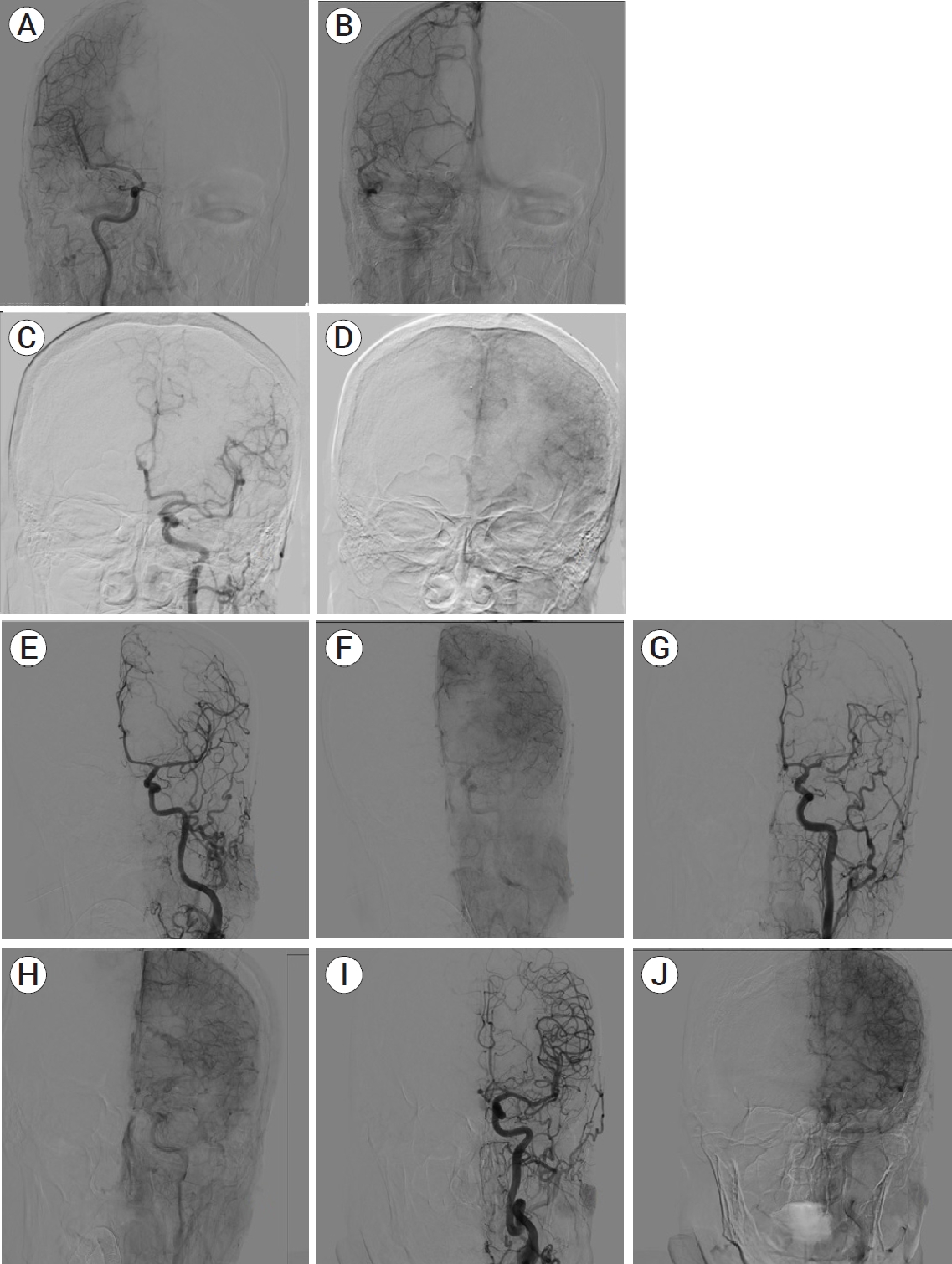
(A) A cerebral angiography of 43 years old male patient with right MCA M1 coilled aneurysm showing right ACA vasospasm without the presence of collateral arteries (ASITN/SIR Grade 0). (B) There is a significant perfusion deficit in the area of the right ACA in subsequent venous phase. (C) 85 years old female patient with ruptured ACA and MCA aneurysm. (D) Cerebral angiography demonstrates vasospasm of left ACA and MCA with slow-flow leptomeningeal collateral vessels with perfusion deficit (ASITN/ SIR Grade 1). (E) 59 years old female patient with coilled right ICA-ACA A1 ruptured aneurysm. Left ACA vasospasm with rapid left MCA leptomeningeal collaterals (ASITN/SIR Grade 2). (F) Mild perfusion deficit can be seen in the subsequent venous phase. (G) 32 years old male patient with ruptured left MCA bifurcatio aneurysm treated with coil embolization. Cerebral angiography demonstrates a severe vasospasm of MCA with rapid ECA leptomeningeal collateral vessels (ASITN/SIR Grade 3). (H) In late venous phase no associated perfusion deficit is detected. (I) 62 years old male patient with ruptured AcomA aneurysm. (J) The cerebral angiography demonstrates vasospasm of ACA with complete and rapid collaterals of MCA leptomeningeal collateral vessels (ASITN/SIR Grade 3). There is no perfusion defisit in the area of vasospasm. MCA, middle cerebral artery; ACA, anterior cerebral artery; ASITN/SIR, American Society of Neurointerventional and Therapeutic Neuroradiology/ Society of Interventional Radiology; ICA, internal carotid artery; ECA, external carotid artery; AcomA, anterior communicating artery
The primary outcome was the association of collateral status with the degree of vasospasm and DCI development both in aneurysmal and non aneurysmal SAH patients. The secondary outcome was the early mortality rate during hospitalization in SAH patients with DCI. The study was approved by the local ethics committee of our university.
Statistical analyses
All statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Group comparisons were performed using statistical methods. We used the Pearson Chi-Square test to compare qualitative data and the Student t-test to compare continuous variables. P-values of less than 0.05 were considered to indicate statistical significance.
RESULTS
A total of 59 patients (21 females and 38 males) with a mean age of 56.38±1.84 (21-82) years were included in the study. We compared patients with bad (grade 0, 1, 2) (n: 38, 64.4%) and good collateral scores (grade 3, 4) (n: 21, 35.6%) and found that DCI was seen more frequently in patients with bad collateral circulation (n: 25 (65.78%) vs n: 7 (33.3%), p: 0.034). There was no statistically significant difference between the good collateral and the poor collateral groups in terms of demographics, baseline Fisher score, occurrence of vasospasm and mortality (Table 1). We also compared SAH patients with (n: 32, 54.24%) and without DCI (n: 27, 45.76%). Patients with DCI were the ones having more severe vasospasm (p: 0.031) and had bad collateral status (p: 0.034). Fisher scores were also found higher in SAH patients with DCI (3.28±0.68 vs 2.55±0.69, p: 0.0001). The cerebral aneurysms had been seen more frequently in SAH with DCI (n: 20 (62.5%) vs n: 7 (25.92%), p: 0.011) (Table 2).
Then the patients were classified as aneurysmal (n: 31, 52.54%) and nonaneurysmal SAH (n: 28, 47.45%). Demographic, hemorrhage severity as Fisher score and DSA data were all compared. Aneurysmal SAH patients had higher Fisher scores (3.4±0.57 vs 2.53±0.67, p: 0.0001) and more frequent DCI (n: 20 (74.07%) vs n: 12 (37.5%), p: 0.011). Mortality rate, collateral status grade, cerebral vasospasm grade scores were all similar (Table 3).
DISCUSSION
Cerebral vasospasm and its possible deleterious effects like DCI should be considered in SAH patients. Although the cerebral collateral system in acute ischemic stroke has been extensively investigated, its impact on SAH patients with DCI is unclear. In such people, the presence of strong collaterals may prevent DCI. According to our data, DCI was more frequently observed in patients who had poor collaterals. Although there was a connection between collateral status and DCI, there was no difference in the severity of vasospasm between the groups with good and bad collateral. In literature there is limited data. Recently Al-Mufti et al. compared aneurysmal SAH patients with and without good collateral circulation and found that patients with severe vasospasm were more likely to have good collaterals (62% vs 33%, p=0.023). In case of mild vasospasm collateral grade was lower (37% vs 9%, p=0.007) [1].
Although their data suggested that severity of vasospasm was associated with the development of collaterals, collateral grades had no effect on DCI which is different from our findings. This might be the result of the severe vasospasm enabling collaterals easier to visualize. Severe vasospasm was seen in over half of the patients, which may have an effect on collateral scoring. There are some differences from our study such as only aneurysmal SAH patients were included and control DSA was performed in 5-7 days after SAH which is earlier than our control angiography. Both aneurysmal and nonaneurysmal SAH patients were included in our study. Unlike the other study, most of our patients had mild to moderate vasospasm [1]. These conflicting results may be due to different pathogenesis of hemorrhages, and methodological differences.
Our findings are compatible with a study including children with SAH. Moftakhar et al. studied cerebral vasospasm in SAH in childhood and found that children with robust cerebral collateral blood flow rarely developed neurological symptoms due to cerebral vasospasm [18]. There is also a study about an experimental model of SAH in rabbits. In this animal model vasospasm with better outcomes seemed to be related with good collaterals in comparison with absent collaterals [7]. Both studies had shown that collaterals have effects on clinical deterioration due to cerebral vasospasm in SAH.
On the other hand the pharmacological agents that reduce proximally seen vasospasm have not been shown to improve outcomes in DCI and agents reducing the rates of moderate to severe vasospasm couldn’t have been shown to improve outcome yet [16,17]. In the mechanism of vasospasm it is well known that endothelial dysfunction and apopitosis plays an important role. The disruption of the endothelial cells due to apopitosis allows for the acute rise in cerebral oedema [3,11] which contributes to the rise in intracranial pressure (ICP) [5]. This results in acute vasoconstriction which, when combined with the oedema, leads to an ischemic injury [5]. Therefore not only collateral circulation but also increased ICP, endothelial dysfunction, apopitosis and brain oedema may affect DCI which should be studied in further studies. The modality used for the diagnosis of cerebral vasospasm is another important point. In spite of the fact that cerebral angiography is the still gold standart, DCI can be a result of distal vasospasm which cannot be accurately diagnosed. All these inconsistencies may be the result of different methodologies of the studies. In order to understand the underlying mechanism of DCI and vasospasm, there should be more detailed larger studies.
DCI was more common in our patients with aneurysmal SAH and patients with higher Fisher scores. Although vasospasm severity didn’t show any statistical difference between aneurysmal and nonaneurysmal SAH patients, DCI was more commonly seen in patients with aneurysm who also had higher Fisher scores demonstrating higher hemorrhagic blood volume.
We found more severe hemorrhages in the aspects of Fisher scores in patients with aneurysmal SAH. It is known that patients with nonaneurysmal SAH have a better clinic than those with aneurysm related SAH [13,25]. Cerebral aneurysm may worsen the amount of bleeding, therefore, aneurysmal cases have more blood, which is associated with worse clinical status with higher Fisher scores [29]. The severity of bleeding may enhance the neuroinflammatory response and endothelial dysfunction. An upregulation of inflammatory cytokines result in endothelium membrane damage, resulting in endothelial cells detaching from the basal lamina. Exposed collagen IV of the basal lamina increases binding and aggregation of platelets and neutrophils that could possibly form microthrombi or microemboli which may contribute to the development of DCI [2,8].
The exact underlying mechanism of DCI is still uncertain and likely multifactorial and interdependent. Recent studies have focused on spasmogenic and neuroinflammatory biochemical factors elevated just acutely after SAH. These substances such as serotonin, arginine vasopressin (AVP) [20] and hypoxia inducible factor-1 (HIF-1) [21] plays important role in SAH pathogenesis. Additionally an alteration of the endothelial nitrous oxide pathway [22] and endothelin-1 receptor activation [24], and pro-inflammatory cascades involving IL-6, IL-1, and tumor necrosis factor-alpha have been shown to be critical in the development and maintenance of cerebral vasospasm following SAH [26]. There is also data suggesting that cerebral vasospasm may in part be mediated by a central control mechanism acting through the sympathetic nervous system [23]. DCI and vasospasm seem to be a result of different pathophysiological factors.
The other important point was possible relationship between cerebral perfusion and vasospasm. In SAH increased ICP inevitably leads to a fall in the cerebral perfusion pressure (CPP), causing global ischemia. In recent studies apoptosis in the vasculature has been found an important factor in SAH leading apoptotic cascades which may be responsible for vasospasm [22]. A good collateral circulation is believed to be one of major important factor for diffuse global ischemia. We believe that collateral circulation, cerebral vasospasm and DCI in SAH have a complex relationship.
There are also limitations of our study. First of all our study group isn’t so large. It is a small cohort of only 59 patients with varied etiologies causing heterogeneity in the study design. We tried to include similiar number of aneurysmal/nonaneurysmal SAH patients with and without DCI for comparison of the groups, which restricted our study. In larger homogenous series DCI, collaterals and vasospasm can be studied and more definite results can be obtained in detailed subgroups. Transcranial doppler (TCD) can be also performed to evaluate patients not only for cerebral perfusion and vasospasm but also intracranial pressure which may be also contribute pathophysiology of DCI. Furthermore cerebral perfusion studies may include more data to understand better the underlying mechanism of DCI. The location, size and also treatment strategies may also affect vasospasm. In patients with no or mild vasospasm, collaterals might not be visualized adequately. Therefore further studies including only SAH with moderate or severe vasospasm would be of great interest. The Hijdra sum score [12] which has previously been correlated with risk of vasospasm in aneurysmal SAH can be also used in further studies with larger study groups. In spite of all these limitations we believe that our study demonstrates the importance of cerebral collateral circulation in cerebral hemodynamics contributing the pathophysiology of DCI which is one of the main prognostic factors for disability after SAH.
CONCLUSIONS
In conclusion, patients with more severe vasospasm and poor cerebral collateral circulation developed DCI more frequently. DCI, on the other hand, was more frequently observed in aneurysmal SAH. Higher Fisher scores in patients with aneurysm and DCI indicated that the intensity of the hemorrhage may be a factor in the pathophysiology. The development of DCI in SAH is thought to be affected by cerebral hemodynamics and hemorrhagic blood volume, hence larger study groups are necessary to fully elucidate the factors delineated in this study.
Acknowledgements
We analyzed the data from our hospital data base and consent of application was not applicable.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.