Surgical considerations and techniques using intraoperative indocyanine green angiography for ethmoidal dural arteriovenous fistula
Article information
Abstract
Objective
This study aims to investigate the efficacy of microsurgery with intraoperative indocyanine green (ICG) angiography as a treatment approach for ethmoidal dural arteriovenous fistula (DAVF).
Methods
Between January 2010 and July 2021, our institution encountered a total of eight cases of ethmoidal DAVF. In each of these cases, microsurgical treatment was undertaken utilizing a bilateral sub-frontal interhemispheric approach, with the aid of intraoperative ICG angiography.
Results
ICG angiography identified bilateral venous drainage with single dominance in four cases (50%) of ethmoidal DAVF, a finding that eluded detection during preoperative transfemoral cerebral angiography (TFCA). The application of microsurgical treatment, in conjunction with intraoperative ICG angiography, resulted in consistently positive clinical outcomes for all patients, as evaluated using the Glasgow Outcome Scale (GOS) at the 6-month postoperative follow-up assessment; six patients showed GOS score of 5, while the remaining two patients attained a GOS score of 4.
Conclusions
The use of intraoperative ICG angiography enabled accurate identification of both dominant and non-dominant venous drainage patterns, ensuring complete disconnection of the fistula and reducing the risk of recurrence.
INTRODUCTION
Dural arteriovenous fistulas (DAVFs) account for 10-15% of all intracranial vascular malformations. Within DAVFs, ethmoidal DAVFs in the anterior cranial fossa make up approximately 10% of cases. Ethmoidal DAVFs are associated with a higher incidence of intracranial hemorrhage, ranging from 62% to 91% [7]. Blood supply to ethmoidal DAVFs typically originates from the anterior or posterior ethmoidal artery, branches of the ophthalmic artery, although one side usually dominates. Occasionally, additional supply may come from branches of the external carotid artery, such as the superficial temporal, middle meningeal, or internal maxillary artery [9,11].
Ethmoidal DAVFs typically drain unilaterally through a cortical drainer into the superficial venous system (superior sagittal, inferior sagittal, spheno-parietal, or cavernous sinus) or the deep venous system [3]. In rare cases, an ethmoidal DAVF may have bilateral drainage, with a dominant, large cortical drainer on the ipsilateral side and a very small cortical drainer on the contralateral side. Although bilateral cortical drainage may occur, the main drainage predominantly occurs through a single dominant drainer, making it challenging to preoperatively detect the non-dominant, small drainer. Therefore, confirming complete disconnection of all cortical drainers, including the contralateral small occulted one, using intraoperative indocyanine green (ICG) angiography is crucial to prevent potentially recurrent residual fistulae.
MATERIALS AND METHODS
Between January 2010 and July 2021, our institution treated eight cases of ethmoidal DAVF. Microsurgical treatment was performed using a bilateral sub-frontal interhemispheric approach, aided by intraoperative ICG angiography. We conducted a comprehensive analysis of the lesion characteristics, the effectiveness of intraoperative ICG angiography, and the clinical outcomes of the patients. The average follow-up period was 20.2 months. Pre- and postoperative assessments included magnetic resonance imaging (MRI), computed tomography angiography (CTA), and conventional transfemoral cerebral angiography (TFCA) with three-dimensional (3D) imaging to facilitate microsurgical planning and evaluate the radiological outcomes of the treatment. Glasgow Outcome Scale (GOS) was used to evaluate each patient’s outcome at 6 months postoperatively.
During surgery, intraoperative ICG angiography enabled the identification of both the ipsilateral arterialized large venous drainer and the traditionally challenging-to-detect contralateral small drainer. Subsequently, complete disconnection of all bilateral cortical drainers was achieved, and coagulation of the mesh engorged dural nidus in the cribriform plate was performed. The effectiveness of the disconnection was confirmed through repeated intraoperative ICG angiography, ensuring complete separation of the fistula from the draining vessels.
RESULTS
The characteristics of the eight cases are summarized in Table 1. Frontal cortical venous reflux was observed in all eight cases, and intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) were observed in four cases (50%). Bilateral ethmoidal arterial feeders, consisting of one dominant feeder and one minor feeder, were present in all cases. In four cases, a single venous drainer was identified, while in the remaining four cases (50%), bilateral venous drainers, with one predominant drainer and one minor drainer, were observed. External carotid arterial feeders were found in one case, requiring division of the sagittal sinus and falx. Superficial venous drainage was observed in seven cases (87.5%). All patients experienced complete recovery, and no complications were reported. Postoperative TFCA revealed complete exclusion of the DAVF in all patients. Among those initially presenting with ICH and/or SAH, two achieved a GOS 4 at the six-month follow-up, while the remaining six patients had satisfactory outcomes with a GOS 5. No recurrences were observed during an average follow-up period of 20.2 months.
Preoperative radiologic findings
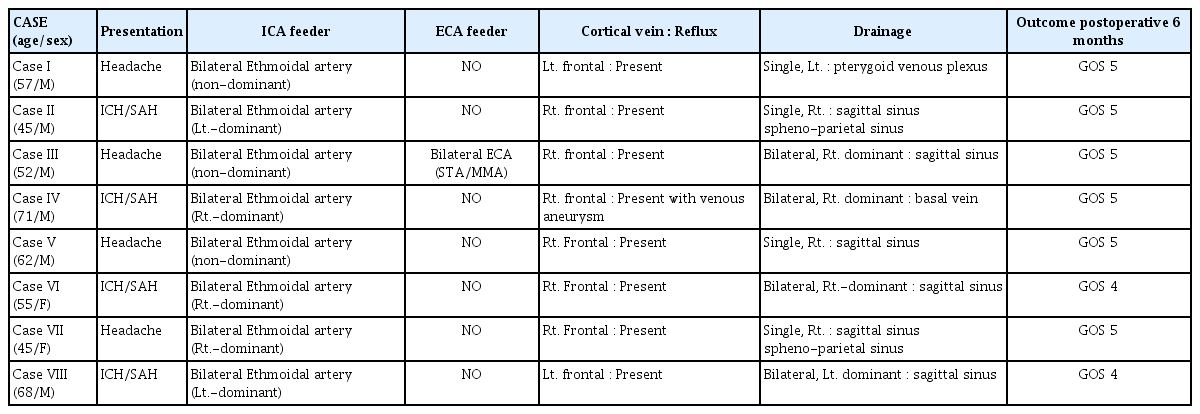
Among the eight cases, four presented with various types of intracranial hemorrhage, including ICH and SAH. Ruptured cases were usually characterized by ICH on the dominant fistula side with SAH (Fig. 1A, Case II in Table 1). On T2-weighted MRI, a prominently dilated venous drainer with a dark signal intensity on the surface of the frontal lobe was consistently observed. Detailed visualization of the DAVF anatomy was achieved through 3D angiography (TFCA or CTA), revealing multiple feeders originating from the ethmoidal arteries, which supplied the DAVF bilaterally with unilateral dominance or without dominance. These feeders traversed the cribriform plate and orbital roof to enter the skull (Fig. 1B, Case II in Table 1). The DAVF exhibited a complex network of engorged vessels within the dura mater covering the cribriform plate. In all eight cases, a single dilated venous drainer was identified draining into either the superficial or deep venous system, while a second smaller contralateral venous drainer remained undetectable in four cases (50%), preoperatively.
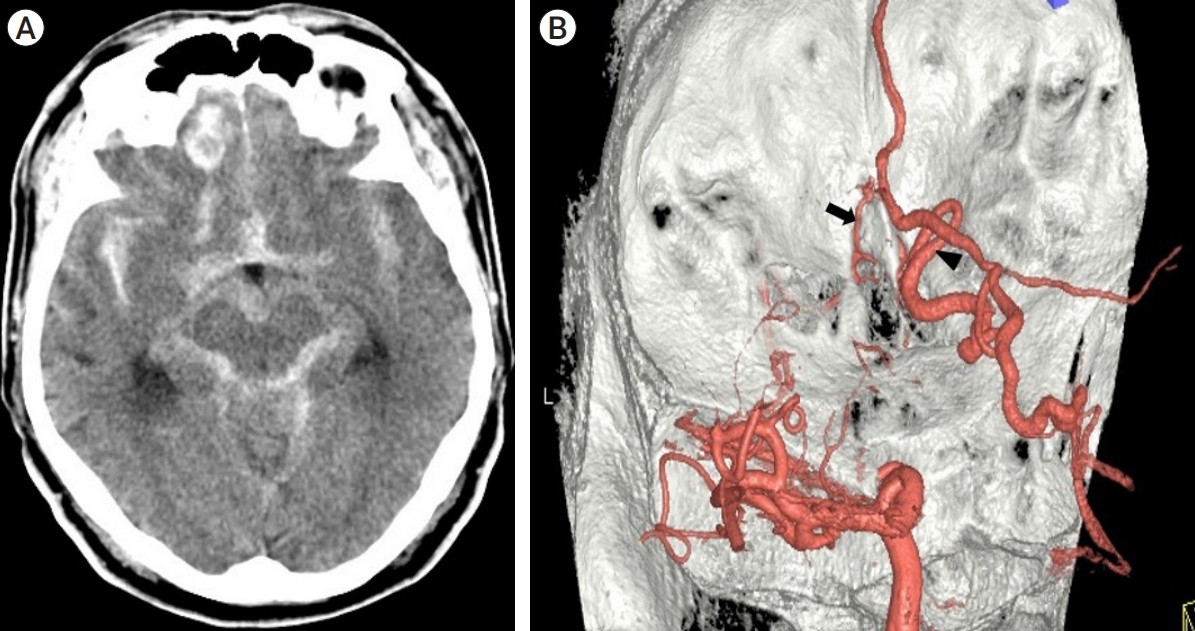
(Case II in Table 1) (A) A 45-year-old man presented with intracerebral and subarachnoid hemorrhage on the computed tomography (CT). (B) A posterolateral left view of a three-dimensional transfemoral cerebral angiography (TFCA) displays an ethmoidal feeder (black arrow) and the main arterialized venous drainer (arrowhead).
Microsurgical operative procedure and intraoperative ICG angiography findings
The representative surgical procedures and intraoperative ICG angiography findings are illustrated in Fig. 2 (Case III in Table 1). A bilateral sub-frontal interhemispheric approach was used for all cases. The dura mater was opened in a “U” shaped manner, parallel to the orbital ridge. During intraoperative ICG angiography, the main arterialized venous drainer, distinguished by its reddish color and the presence of a pulse, was easily identified (Fig. 2A). It is not easy to distinguish because of its small size, but a strongly suspected non-dominant small venous drainer was observed under microscopic view (Fig. 2B). Bilateral venous drainage, which was not identified in the preoperative TFCA, was also confirmed in four cases through intraoperative ICG angiography. The vessel suspected to be a small non-dominant drainer under the microscopic view, referred to in Fig. 2B, also confirmed early filling during the arterial phase like dominant main venous drainer (Fig. 2C). Following the identification of the primary dominant drainer, a clipping technique was applied at a location proximal to its origin from the dura mater. The nidus within the cribriform plate was then completely coagulated using bipolar coagulation. These surgical maneuvers resulted in a progressive change of the main drainer’s color to a bluish hue and a gradual decrease in pulse intensity. Additionally, the small non-dominant venous drainer, identified in 4 out of 8 cases through intraoperative ICG angiography, was also disconnected by coagulation (Fig. 2D). Subsequent intraoperative ICG angiography confirmed the complete obliteration of the ethmoidal DAVF (Fig. 2E).
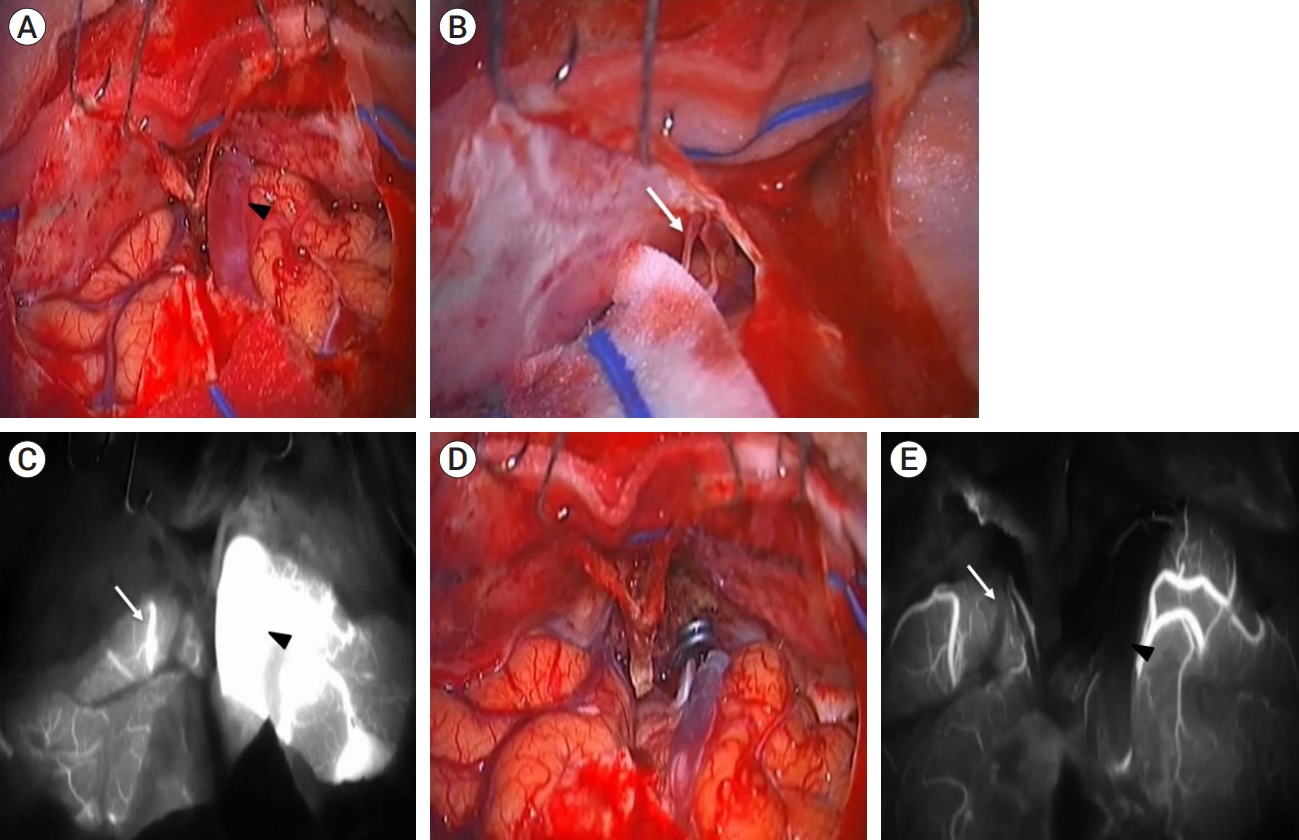
(Case III in Table 1) All figures were intraoperative microscopic findings, and the surgery was performed with an interhemispheric approach in the supine position. (A) The main arterialized venous drainer, which was identified in the preoperative TFCA, is observed (black arrowhead). (B) Blood vessels, suspected to be the contralateral small non-dominant venous drainer, are observed under the microscopic view (white arrow). (C) Intraoperative ICG angiography confirmed bilateral venous drainage, not only the main arterialized venous drainer (black arrowhead) but also the small non-dominant venous drainer (white arrow), which was not observed in the preoperative TFCA and was suspected under microscopic view. (D) Once the primary dominant drainer was defined, it was clipped just proximal to its origin at the dura mater. Subsequently, the nidus within the cribriform plate was completely coagulated using bipolar forceps. These surgical maneuvers led to a gradual change in the color of the main drainer, which turned bluish, and a decrease in pulse intensity. Furthermore, the small non-dominant venous drainer, which was only identified during intraoperative ICG angiography, was also disconnected by coagulation. (E) After disconnection, ICG angiography was performed again, confirming complete obliteration of both the main dominant venous drainer (black arrowhead) and the small non-dominant venous drainer (white arrow), which were observed in the early arterial phase during previous ICG angiography. Following complete disconnection, neither the dominant nor the non-dominant venous drainers were detected in the early arterial phase anymore, and they appeared non-pulsatile and pale. TFCA, transfemoral cerebral angiography; ICG, indocyanine green
Although a minor contralateral venous drainer could not be identified preoperatively in any of the eight cases, repeated ICG angiography revealed the presence of a minor drainer in four cases (50%). In these cases, the small venous drainers were also coagulated. Before complete closure of the operative wound, the opened frontal sinus was sealed with a prepared periosteal layer after removing the frontal sinus mucosal membrane. This was done to ensure a water-tight closure of the dura mater, which is crucial in preventing postoperative complications such as cerebrospinal fluid rhinorrhea, mucocele formation, and infection.
DISCUSSION
A DAVF is a rare cerebrovascular disease characterized by an abnormal connection between arteries and veins bypassing the capillary bed within the dura mater, the outermost membrane surrounding the brain and spinal cord [4]. The DAVF accounts for 10-15% of all cerebral vascular malformation. Among them, DAVF occurring in the anterior cranial fossa represents about 10% of cases. In other words, anterior cranial fossa DAVF, also known as ethmoidal DAVF, is an extremely rare condition, comprising only 1-1.5% of the total cerebral vascular malformations [5].
The majority of DAVFs are supplied by an ophthalmic artery ethmoidal branch. Bradley A. Gross et al. reported that 93% of ethmoidal DAVF cases had a bilateral supply from this branch, and 48% of cases had a bilateral supply from the distal internal maxillary artery ethmoidal branch [6]. Most ethmoidal DAVFs have cortical vein drainage, so they are classified as grade III or IV in the Cognard classification. Kan Xu et al. reported that all 48 cases were classified as a high-grade due to the cortical venous drainage. Typically, approximately 70% of ethmoidal DAVFs are drained to the anterior of the superior sagittal sinus (SSS), around 30% are drained to the posterior, and 20% of them are drained into the deep vein mainly the basal vein of Rosenthal [6,13]. These venous drainages are typically unilateral, with bilateral drainages being extremely rare [8,13]. In our study, all eight cases had bilateral ethmoidal arteries as feeding arteries, and all cases exhibited cortical venous reflux. Six out of the eight cases were drained by the SSS, while the remaining one was confirmed to be drained by the basal vein. In four cases, TFCA performed preoperatively revealed unilateral venous drainage, but intraoperative ICG confirmed bilateral venous drainage, especially.
Symptoms in DAVF primarily arise from the specific location of the venous drainage. In the case of ethmoidal DAVF, symptoms such as proptosis, chemosis, ophthalmoplegia, and decreased visual acuity are observed. Moreover, patients may also experience additional symptoms such as headache, seizures, dementia and cognitive dysfunction [1,2].
In particular, ethmoidal DAVFs are characterized by cortical venous drainage, which gradually weakens the vein and causes structural deformation. This process is often accompanied by venous ectasia and venous aneurysm, ultimately leading to the occurrence of hemorrhage [13]. Kan Xu’s review reported that the rate of venous ectasia ranged from 46% to 73%. Therefore, urgent treatment for ethmoidal DAVF should be considered regardless of the patient’s current condition.
Throughout the years, multiple treatment modalities have been suggested for ethmoidal DAVF, such as microsurgery, endovascular embolization, radiosurgery, or their combinations.
Although radiosurgery can be a safe and effective treatment option for ethmoidal DAVF, it is not generally regarded as a first-line treatment due to the high risk of bleeding associated with cortical venous drainage and the delayed response time of radiosurgery [1,10]. Additionally, according to a study by Huaiche Yang et al., the obliteration rate for patients treated with radiosurgery alone was lower, at 67%.
While there have been notable advancements in endovascular treatments of the ethmoidal DAVF like microcatheters and balloons in recent years, endovascular embolization still presents certain challenges. In ethmoidal DAVF, technically accessing the feeding artery can be challenging, particularly if it is tortuous. Moreover, there is a risk of visual deficit associated with embolization of the central retinal artery and ophthalmic artery. Furthermore, given the high risk of bleeding in ethmoidal DAVF, it is crucial to aim for complete obliteration to prevent recurrence. However, endovascular embolization exhibits a relatively higher rate of incomplete obliteration compared to surgical disconnection [5,13]. Therefore, endovascular embolization is preferably performed on a limited cases when there is a favorable angiographic anatomy with easy access to the fistular point through a trans-arterial approach. In a review conducted by Stefanos Giannopoulos et al., surgical disconnection was found to be superior to endovascular embolization in achieving complete obliteration (surgery group, 100% [n=65/65]; embolization, 47% [n=15/32]). Microsurgery has been associated with complications such as postoperative hemorrhage, postoperative infection, and cerebrospinal fluid (CSF) leak [12]. However, there was no significant difference in periprocedural 30-day outcomes when compared to endovascular embolization [5]. Moreover, microsurgery offers the advantage of reducing intracranial pressure through the evacuation of hemorrhage, particularly in cases where the lesion has already bled. Intraoperative ICG angiography can enhance the effectiveness of microsurgery in achieving safe and complete occlusion of the fistula. While TFCA is regarded as the gold standard for preoperative diagnosis, it may not identify contralateral small venous drainers in certain patients. These small drainers have the potential to contribute to recurrence because, following the occlusion of the primary drainer, the secondary smaller drainer becomes the exclusive drainage pathway, resulting in increased flow that eventually leads to recurrence. Consequently, meticulous confirmation of existence and disconnection for the secondary smaller drainer through repeated intraoperative ICG angiography before and after occluding the primary venous drainage can aid in effectively identifying and occluding any secondary drainers.
CONCLUSIONS
Microsurgery utilizing intraoperative ICG angiography proved to be effective in the treatment of ethmoidal DAVF, resulting in excellent angiographic and clinical outcomes for all patients. Intraoperative ICG angiography enables accurate identification of both dominant and non-dominant cortical venous drainers, confirmation of complete disconnection of the fistula, and prevention of recurrence.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.