Flow arrest during carotid artery stenting with a distal embolic protection device: A single-center experience and clinical implications
Article information
Abstract
Objective
We aimed to investigate the incidence of flow arrest during carotid artery stenting (CAS) with filter-type embolic protection device (EPD), identify any predisposing factors for those situations, and contemplate intraprocedural precautionary steps.
Methods
CAS was performed in 128 patients with 132 arteries using filter-type EPD. The characteristics of treated patients and arteries were compared between groups with and without flow arrest.
Results
The incidence of flow arrest during CAS with filter-type EPD was 17.4%. In flow arrest group, cases of vulnerable plaques (p=0.02) and symptomatic lesions (p=0.01) were significantly more common, and there were more cases of debris captured by EPD in a planar pattern (p<0.01). Vulnerable plaques were significantly more common in the procedures showing a planar pattern than in the cases with other patterns (p<0.01). Flow arrest group showed a significantly higher rate of ischemic complications (p<0.05), although there were no significant periprocedural neurological changes. The planar pattern of captured debris in filter-type EPD was the only significant risk factor for flow arrest (adjusted odds ratio 88.44, 95% confidence interval 15.21-514.45, p<0.05).
Conclusions
Flow arrest during CAS with filter-type EPD is not uncommon and associated with increased ischemic complications. Symptomatic stenoses and vulnerable plaque are related to this event. The planar pattern of captured debris on the EPD was the only significant risk factor for the flow arrest. Clinicians must pay attention to the occurrence of flow arrest and react quickly when performing CAS.
INTRODUCTION
Carotid artery stenting (CAS) is considered a treatment option for carotid artery stenosis. In addition to the clear benefit of carotid endarterectomy (CEA) for moderate to severe symptomatic and severe asymptomatic carotid artery stenosis compared to the best medical treatment only, which was proven in representative trials [2,8,9], CAS showed noninferiority and similar clinical outcomes compared to CEA and was suggested as an alternative treatment modality for carotid stenosis [3,4,20,25].
To reduce embolic complications during the CAS procedure, embolic protection devices (EPDs) have been applied and are widely used. CAS protected by EPD showed fewer adverse events than CAS protected by the unprotected procedure [13]. EPDs can be divided into distal occlusion balloons, distal filters, and proximal protection systems [10]. Among them, using filter-type EPD, flow impairment or arrest was not uncommon [5] as a result of filter obstruction; moreover, blockage of filters tends to result in cerebral infarction [14]. As part of efforts to reduce complications after CAS, a detailed exploration and review of flow arrest during the CAS procedure is needed.
We aimed to investigate the incidence of flow arrest during the CAS procedure with filter-type EPD, identify any predisposing factors for those situations, and contemplate intraprocedural clinical precautionary steps.
MATERIALS AND METHODS
Patient selection
From March 2012 to July 2022, a total of 219 patients with 229 arteries were treated by CAS in our institution. Among them, some patients were excluded for the following reasons: 8 patients underwent CAS as a combined procedure with mechanical thrombectomy for acute ischemic stroke; 78 patients were missing the results of laboratory studies; one patient did not use the filter-type EPD; and 4 patients underwent bilateral CAS at two separate sessions (for ease of statistical analysis). Finally, 128 patients with 132 arteries were selected as final candidates for our study.
The procedure of carotid stenting
All CAS procedures were performed under local anesthesia in a neuroangiographic suite using a biplane angiographic machine (Allura Xper and Azurion, Philips Medical Systems, Best, the Netherlands). All patients were administered dual antiplatelet agents (acetylsalicylic acid and clopidogrel) at least 2 weeks before the procedure. A 6F long sheath (internal diameter) was inserted through a puncture of the femoral artery, and 3000 international units of heparin were intravenously bolus injected. In all procedures, we used filter-type EPD and tried to pass the EPD through the stenotic segment. Emboshield (Abbott Vascular Devices, Redwood City, CA, USA) was mainly used (in 131 procedures), and in only one procedure, Spider (EV3, Plymouth, MN, USA) was used. After the deployment of EPD, percutaneous transluminal angioplasty (PTA) was performed by inflation of non-compliant balloons. Except for only one case using a balloon-mounted stent, self-expandable stents were implanted in all cases. Post-stent ballooning was not performed routinely. EPD was withdrawn by retrieval catheter, and the inside of the filter was grossly inspected after irrigation by nonheparinized saline. All intraluminal manipulations in CAS procedures proceeded under digital roadmap images, and angiography was performed as a final step to confirm that stenosis was resolved properly, the stent implantation was adequate, the flow run-off was improved, and that any thromboembolism existed in the intracranial vasculature. Dual antiplatelet medication was maintained for at least 6 months after CAS.
Data collection
Electronic medical records of patients were reviewed retrospectively to check the patients’ age, sex, underlying diseases, current smoking status, various lab findings, and any neurologic symptoms related to treated arteries before the CAS procedure. Among lab findings, measurement of residual platelet reactivity was performed by VerifyNow P2Y12 assay (Accriva Diagnostics, San Diego, CA, USA), providing the values of aspirin reaction unit (ARU), P2Y12 reaction unit (PRU), and percentage inhibition. ARU above 550 indicates resistance to acetylsalicylic acid, and high PRU and low percentage inhibition indicate high residual platelet reactivity and poor effectiveness of thienopyridine antiplatelet medications, and vice versa. We reviewed 2D angiography and 3D rotational angiographic images in the Picture Archiving Communicating System (PACS) to confirm the location and degree of stenoses by North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria [11] and plaque ulceration. To express the nature of the stenotic segment in a more meaningful way, the ratio of the degree of stenosis and the longitudinal length of the stenotic segments was obtained as a percentage, and these values were designated the stenosis-length ratio (SLR). Plaque ulceration was defined as a case in which the connection of contrast to plaque extended beyond the continuous vascular lumen was observed in 2D or 3D rotational angiography [15]. Vulnerable plaque was defined as follows: carotid ultrasonography (USG) showed hypoechoic predominant plaques [19], and the vessel wall (VW) magnetic resonance images (MRI) showed ulcerative surface and/or thin fibrous cap and/or lipid core and/or intraplaque hemorrhage [21]. Procedure time for CAS (by minutes) and degree of improvement in stenoses by percentage ([initial degree of stenosis - degree of remaining stenosis after CAS]/initial degree of stenosis) were also checked. Preprocedural brain MRI, including diffusion-weighted imaging (DWI), was reviewed to confirm cerebral infarction, and its patterns were subclassified as embolic, borderzone, and mixed types. If flow arrest occurred during the procedure, it was confirmed at what point during the procedure steps flow arrest occurred, and in such cases, the patterns of debris captured by EPD were classified into three types: no debris; scattered, aspects of only a small amount dispersed inside the filter; and planar, covering more than one side of the filter. Fig. 1 shows typical examples of those patterns. Any complications during or after CAS were identified and collected. Finally, neurologic status before CAS and one month after treatment was evaluated by the modified Rankin Scale (mRS) [1].

The gross pictures for the examples of patterns of captured debris on filter-type embolic protection devices. (A) shows the scattered pattern, and (B) demonstrates a planar pattern of debris, which was the case of flow arrest.
Our institutional review board approved the study protocol (IRB No. 10-2023-24). The requirement to obtain written informed consent for study participation was waived. Data are publicly available upon reasonable request.
Statistical analysis
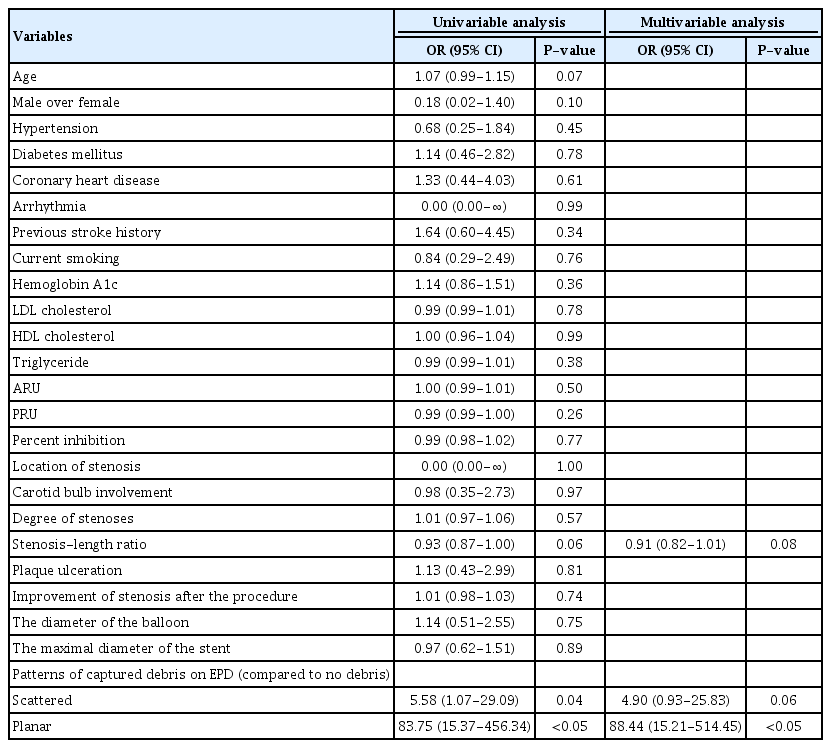
Continuous variables are presented as the mean±-standard deviation (SD), and comparisons between the groups of flow arrest and no flow impairment during CAS were performed by Student’s t-test or Mann‒Whitney test. Categorical variables were expressed as numbers and percentages of total cases, and comparisons between the groups of flow arrest and no flow impairment during CAS were performed by the chi-square test or Fisher’s exact test, or linear by linear association. The test of normality was performed by the Shapiro‒Wilk method. The Wilcoxon signed-rank test was used to compare neurological status before and after the CAS procedure. Risk factor analysis was performed by logistic regression to produce the odds ratio (OR) with a 95% confidence interval (CI) for the risk factor evaluation of flow arrest. Variables with a p-value >0.2 in univariable analysis and deemed fundamental factors, such as age or sex, and some underlying disease of cerebral atherosclerosis were selected for multivariable analysis in a backward elimination. For all statistical tests, a p-value <0.05 was considered statistically significant. All statistical tests were performed using SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA).
RESULTS
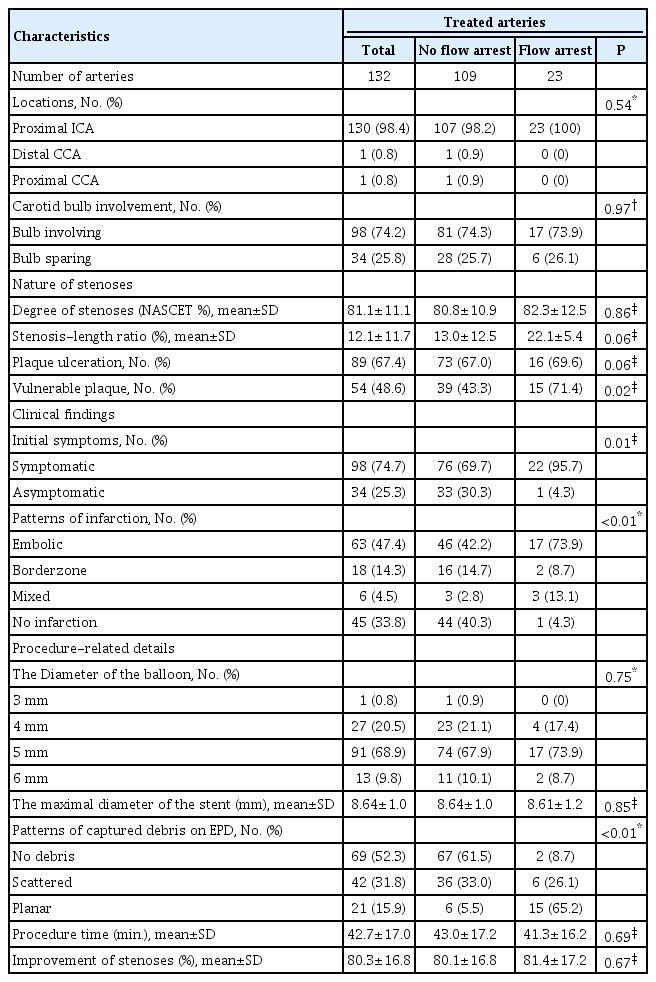
The baseline characteristics of the 128 patients in this study are described in Table 1. The patient population was predominantly male (82.8% of total patients), and the mean age was 72.8 years. The most common underlying disease was hypertension. Except for patient age and current smoking status, there were no significant differences in other characteristics between the groups with and without flow arrest during CAS.
The details of the characteristics of the treated stenoses are presented in Table 2. The incidence of flow arrest during CAS was 17.4%. The location of almost all treated arteries was the proximal internal carotid artery (ICA), and it showed a nearly even distribution. Stenoses involving the carotid bulb were approximately three times more common than cases that spared the bulb. The mean degree of stenosis was 81.8% (by NASCET criteria), the mean value of SLR was 12.1%, and a higher SLR was observed in the flow arrest group with borderline significance. Plaque ulceration was observed in more than half of the total treated arteries and was more common in the cases of flow arrest with borderline significance. Except for 21 arteries for which plaque vulnerability was not properly evaluated because carotid USG or VW MRI were not performed, a total of 37 lesions were evaluated for plaque vulnerability using both carotid USG and VW MRI, 68 lesions using carotid USG alone, and 6 lesions using VW MRI alone. There were 6 cases of flow arrest among the 31 arteries identified as having a vulnerable plaque on carotid USG, 9 cases among the 19 arteries identified as having a vulnerable plaque on VW MRI, and none among the 4 lesions identified as having a vulnerable plaque on both carotid USG and VW MRI. The vulnerable plaque was significantly more common in the flow arrest group than in the arteries without flow arrest (p=0.02). The rate of symptomatic lesions was significantly higher in the flow arrest group than in the no flow arrest group. In symptomatic stenoses, limb weakness was the most common clinical presentation. In the cases of positive radiological findings for ischemic events, embolic cerebral infarction was the majority of DWI patterns, but the cases that had no radiologic evidence of ischemic events were also the majority of the no flow arrest group. On the other hand, embolic infarction was a significantly and overwhelmingly dominant pattern of infarction in the flow arrest group. The diameters of PTA balloons were from 3 to 6 mm, and the 5 mm-sized balloon was used the most frequently. There was no significant difference in not only the balloon but stent size used between the two groups with and without flow arrest. Most of the debris was captured with a planar pattern by EPD in the cases of flow arrest (65%), whereas the majority of procedures without flow arrest did not present any captured debris in the filter of EPD (61.5%), presenting a difference that showed significance (p<0.01). There were no significant vasospasms in the two cases in which no debris was captured by the EPD in the flow arrest group. Limited to cases where plaque vulnerability could be assessed, vulnerable plaques were significantly more common in 77.8% (14/17) of the procedures showing a planar pattern of captured debris than in 43.0% (40/93) of cases with other patterns (p<0.01 by chi-square test, not shown at tables). The mean time of the CAS procedure and mean improvement of stenoses showed no significant differences between the two groups with and without flow arrest.
Table 3 summarizes 23 cases of flow arrest that occurred during CAS. More than half of flow arrest occurred after PTA. In one case, during the passage of the EPD through a severely stenotic segment, the atherosclerotic plaque was ruptured, which led to flow arrest. The most common complication in the flow arrest group was embolic cerebral infarction (17.4%), and there were also two cases of branch retinal artery occlusion (BRAO). Intra-arterial thrombectomy using the suction aspiration technique was performed for intracranial large artery occlusion (middle cerebral artery M1 occlusion) in one case. Except for adverse events not related to cervical or intracranial arteries, such as puncture site infection, the complication rate in the no flow arrest group was 3.7%, whereas it was 29.1% in the flow arrest group, and this finding showed statistical significance (p<0.01 by Fisher’s exact test, not shown at tables). When we compared mRS scores before and 1 month after CAS in individuals with flow arrest, we found that there was no significant difference (p=0.19). One mortality case was not a complication directly related to the procedure but death from massive intracerebral and intraventricular hemorrhage due to excessive prolongation of activated partial thromboplastin time (aPTT) during heparinization for corona radiata infarction that occurred after the CAS procedure. In individuals without flow arrest, there were 7 cases of procedure-related complications (two for cerebral infarction within 1 month after CAS, three for puncture site problems, and one for iatrogenic carotid dissection). Comparing the rate of thromboembolic complications between the two groups with (34.8%) and without (1.8%) flow arrest during CAS, it was found that the flow arrest group showed a significantly higher rate of ischemic complications related to procedures (p<0.05 by Fisher’s exact test, not shown in tables). The overall rate of complications related to procedures, not just those with flow arrest, was 12.9%. The details of overall complications are presented in Supplementary Table 1.
The results of the analysis for risk factors of flow arrest during the CAS procedure are shown in Table 4. In multivariable analysis, the planar pattern of captured debris in EPD was the only significant risk factor (OR 88.44, 95% CI 15.21-514.45, p<0.05).
DISCUSSION
In this study, within a single institution, the incidence of flow arrest during CAS using filter-type EPD was 17.4%, most of the events (69.6%) occurred immediately after PTA, and the embolic complication rate was significantly higher than in the case of no flow arrest, but there was no significant difference in clinical outcomes before and after CAS. The planar pattern of captured debris on the EPD was the only risk factor for the occurrence of flow arrest during CAS. Unlike other studies on similar subjects [5,7,17,18], we almost always used a single type of filter-type EPD, and post-stenting PTA was not performed.
In our institution, CEA was used in place of CAS when the primary component of the stenotic lesion is a very hard, calcified plaque, which is not appropriate for CAS. The majority of individuals in this study were elderly and had cardiopulmonary functions that were intolerant to general anesthesia, so CAS was chosen over CEA for carotid stenosis in addition to patients with vulnerable plaque.
Distal embolization of debris generated during endovascular procedures is known as the most important acute complication of CAS [16]. To reduce this adverse event, numerous studies comparing patients treated with and without EPD were performed. In a meta-analysis, Garg et al. showed a significantly lower risk of stroke in protected procedures than in unprotected procedures (relative risk 0.62, 95% CI 0.54-0.72) [12]. Flow impairment during carotid intervention using filter-type EPD was thought to occur because particulate debris containing plaque elements such as fibrin, lipid-rich macrophages, and cholesterol results in blockage of the filter pores [5,17]. This flow impairment was known to be associated with an adverse clinical outcome, such as the 30-day incidence of stroke or death (9.5% vs. 2.9%, p=0.03) [5]. Previous studies reported the incidence of flow impairment during CAS with EPD as 9.8 to 31% [5,6,17], and our observed incidence of flow arrest (17.4%) was also comparable to those results. Similar to the previous study, flow arrest was related to a higher incidence of ischemic events (34.8% vs. 1.8%, p<0.05). There were no significant periprocedural neurological changes even in cases of flow arrest, but the difference in incidence was much larger, highlighting a caution to not ignore the event of flow arrest during CAS. It also implies that the selection of treatment methods should be carefully thought out.
If a large amount of plaque elements sufficient to block the filter of EPD was generated during endovascular procedures, it was likely to be a relatively vulnerable plaque of carotid stenosis, and we assumed that such a lesion is more likely to be discovered and diagnosed as symptomatic rather than asymptomatic. Compared to the cases of CAS without flow arrest events, the individuals who experienced flow arrest had characteristics such as a higher rate of symptomatic lesions and embolic infarction at initial presentation, mostly scattered or planar patterns of captured debris on EPD, and a higher rate of procedure-related complications, and these findings supported our speculation.
The degree of stenosis and plaque ulceration itself were not significant risk factors for flow arrest, but plaque ulceration and high SLR were more common in the flow arrest group with borderline significance. Not only the degree but also the length of stenosis was reported as a risk factor for thromboembolic complications of CAS in a previous study [23], so we calculated the SLR to create an index that can reflect both the degree and length of stenosis. The higher mean SLR in the flow arrest group than in individuals without flow arrest may be interpreted as meaning that when the high degree of stenosis is concentrated in the relatively short segment, the debris migrates away and accumulates on the filter of EPD because of the much greater atherosclerotic burden. The results showed borderline significance may merely be on account of the number of cases not being so many.
In previous research, severe (≥90%) stenosis of the target lesion [17] was found to contribute to flow impairment during CAS with filter-type EPD, and Casserly et al. reported significant predisposing factors to flow impairment as symptomatic (within 6 months) carotid lesions, larger stent diameter, and increased age [5]. As to the question of whether vulnerable plaque itself is also a risk factor, the results of preceding studies are conflicting. Sakamoto et al. presented vulnerable plaque as a risk factor for flow impairment [22], but other authors showed that vulnerable plaque was not predictive of flow impairment [18]. This disparity may depend on the diagnostic modalities or definition of a vulnerable plaque. Unfortunately, there were no additional significant risk factors for flow arrest except for the planar pattern of captured debris on EPD in this study. Although we showed a higher proportion of vulnerable plaque in the flow arrest group and the relationship between a greater amount of captured debris in the EPD and vulnerability of plaque, we have not been able to perform the risk factor analysis to obtain meaningful results because there were too many missing values (21 cases) due to a lack of any imaging studies that could evaluate plaque vulnerability accurately, such as carotid USG or VW MRI.
To reduce complications related to flow arrest that occur during the CAS procedure, rapid and atraumatic device manipulation can shorten the procedural and ischemic time. Most flow arrest occurred just after PTA, which may suggest that clinicians need to pay attention to the patient’s condition during CAS, especially during PTA, and to perform angiographic evaluation immediately after de-ballooning. There were two cases of no visible captured debris in EPD with flow arrest, and in these individuals, there were no other specific findings, such as vasospasm, that could cause flow arrest. A possible explanation for this is that during the retrieval of the EPD, the filter structure is squeezed, and the debris can escape through the filter pore [17]. This assumption suggests that we need to be more careful and apply gentle manipulation when retrieving the filter-type EPD. Some investigators insisted on a higher incidence (84%) of flow impairment during CAS with filter-type EPD revealed by frame-by-frame comparison of angiographic images compared with the clinician’s classification [24], and that finding means that there may be more frequent flow compromise than we are aware of, and caution is needed during CAS procedures.
There were several limitations in our study. First, this was a retrospective study conducted in a single institution with a small number of individuals. In the classification of captured debris in the EPD, the amount or patterns of captured debris were not measured quantitatively but only grossly. Also, patterns of captured debris on the EPD result from the procedure, not the underlying cause of a flow arrest in the strict sense. Additionally, the vulnerable nature of plaque located at stenosis thought to contribute greatly to the generation of a large amount of debris, was not properly evaluated in all cases.
CONCLUSIONS
Flow arrest during CAS with filter-type EPD is not uncommon and is associated with increased ischemic complications. Symptomatic lesions of stenoses, especially presented as embolic infarction, and vulnerable plaque are related to this event. The planar pattern of captured debris on the EPD was the only significant risk factor for the occurrence of flow arrest. Further well-designed studies are required to investigate other predisposing factors, including the evaluation of plaque vulnerability. Clinicians must pay attention to the occurrence of flow arrest and react quickly when performing CAS.
Supplementary table
Summary of the overall complications in all procedures
Acknowledgements
This work was supported by a general clinical research grant-in-aid from Seoul National University-Seoul Metropolitan Government (SNU-SMG) Boramae Medical Center (04-2023-0022).
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.