Endovascular Treatment of the Distal Internal Carotid Artery Large Aneurysm
Article information
Abstract
Objective
According to the development of endovascular technique and devices, larger aneurysms on the distal internal carotid artery (ICA) can be treated using a less invasive method. The authors report on clinical and angiographic outcomes of these aneurysms treated using an endovascular technique.
Materials and Methods
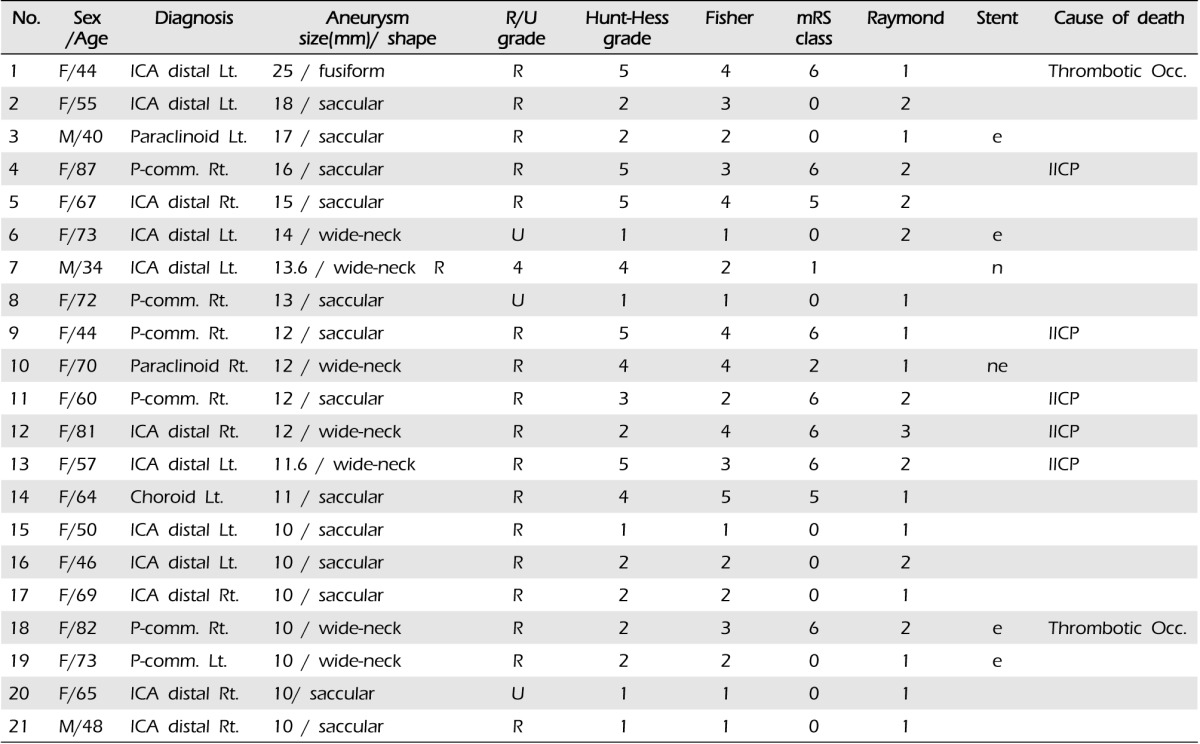
Data on 21 patients with large aneurysms at distal ICA treated by endovascular method between January 2005 and December 2012 were included in this retrospective analysis.
Results
Clinical outcome of patients showed strong correlation with the initial neurologic status (p < 0.05). Aneurysm morphology showed saccular, fusiform, and wide-neck in 12, six and three patients. Six patients underwent stent assisted coiling and the other 15 patients underwent simple coiling. Aneurysm occlusion was performed immediately after embolization with near-complete (Raymond class 1-2) in 20 patients (95.2%) and incomplete (Raymond class 3) in one patient (4.8%). Delayed thrombotic occlusion occurred in two patients and their clinical result was fatal. Another five patients died in the hospital, from massive brain edema and/or increased intracranial pressure due to initial subarachnoid hemorrhage. Overall mortality was 30% (seven out of 21). Fatal complication related to the endovascular procedure occurred in two patients with thrombosis at middle cerebral artery (one with stent, the other without it).
Conclusion
Recent developed endovascular device and technique is safe enough and a less invasive method for distal large or giant aneurysms. Based on our analysis of the study, we suspect that coil embolization of large distal ICA aneurysms (with or without stenting) is effective and safe.
INTRODUCTION
Ideal treatment for cerebral aneurysm is to decrease its mass effect, and maintain the integrity of normal vasculature. It is achieved by surgical clipping across the aneurysm neck or endovascular coil embolization to preserve the parent vessels.17)18)22)26) The above mentioned procedures can be performed in the majority of intracranial aneurysms. However, particularly in large or giant aneurysms, those measures are inapplicable because of specific pathophysiology and anatomical configuration of those types of aneurysm.17) Traditional surgical techniques can be ineffective in cases where the entire aneurysm is included in the parent vessel wall, or its location is near or partially within the cavernous sinus, or its size is too large to interrupt surgical view or to clip in the case of hard atherosclerotic plaque or having no definite aneurysmal neck. Such a condition makes them inappropriate candidates for surgical or endovascular treatment.17) The latest advanced imaging technique, particularly angiography made by 3-dimensional reconstructive imaging, clarifies the configurations of aneurysm and vital perforating or distal branch vessels. In this article, the authors report results of minimal invasive endovascular coiling in 21 patients with a large aneurysm of the distal intracranial artery (dICA).
MATERIALS AND METHODS
Patient characteristics
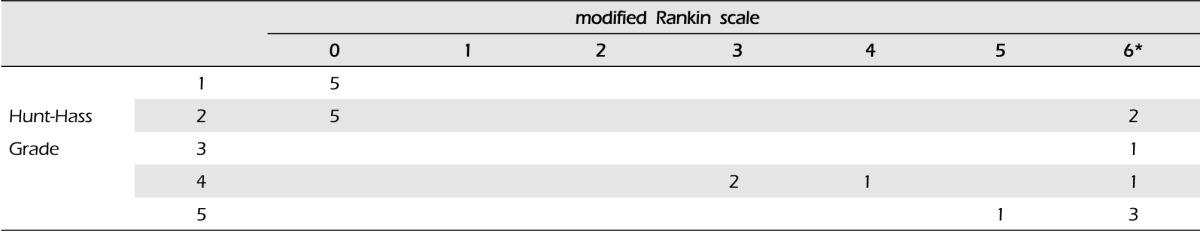
Between January 2005 and December 2012, 453 aneurysms in 442 patients were treated by endovascular coiling with or without stent assisted technique in our institute. Among them, there were 21 patients with large distal ICA aneurysms (longest diameter more than 10 mm), and patients' characteristics are summarized in Table 1. There were three men (14.3%) and 18 women (85.6%) with a mean age of 61.0 years (median 64 years, range 34-87 years). In 21 patients with aneurysms, 16 (76.2%) were ruptured and the other five (23.8%) were unruptured, respectively. According to Hunt-Hess (H-H) grade, there were I-II in 12 patients, III in one, and IV-V in eight. Endovascular treatment was performed within 24 hours after subarachnoid hemorrhage in all 16 patients with ruptured aneurysms. Of the five patients with unruptured aneurysms, four were found incidentally and one presented with symptoms of a mass effect, third nerve palsy. The initial neurological status was measured according to Hunt-Hess Grade and Glasgow coma scale (GCS) scores at the time of arrival in our institute. Clinical outcomes were evaluated according to modified Rankin scale (mRS) scores and measured at 90 days after the initial treatment. Favorable outcome was defined as mRS 0-2, unfavorable outcome mRS 3-6. Overall mortality and cause of death were also recorded.
Radiological evaluations
Radiologic evaluation was performed by a radiologist who did not participate in patient management. On preoperative angiography of the distal ICA aneurysm, we checked neck, width, height, location, daughter sac, morphology (saccular, fusiform), and bifurcation type of the aneurysm. Post-procedure imaging studies were also reviewed for evaluation of complete obliteration, neck remnant, and intra-procedure complication, such as thrombosis, emboli, and perforation. Aneurysm occlusion was estimated according to Raymond classification.
Endovascular technique
All procedures were performed on a biplane angiographic unit (Axiom Artis, Simens Medical Systems, Erlangen, Germany) under general anesthesia. Routine diagnostic 4-vessel angiography was performed. An image of the aneurysm was drawn using 3-D rotational angiography. The procedure was performed using a 7F sheath (Radifocus Introducer II, Terumo, Hatagaya, Japan) using a transfemoral approach. The guide catheter was the Guider Softip (Boston Scientific, Natick, MA, United States). Excelsior (Stryker Neurovascular, Fremont, CA, United States) was used as a microcatheter. Two types of stent, Neuroform stent (Boston Scientific, Natick, MA, United States) and Enterprise (Cordman & Shurtleff, Inc., Raynham, MA, United States) were used in this study. Angiographic outcome was evaluated according to Raymond class as complete obliteration (class 1), residual neck (class 2), and residual aneurysm (class 3). Immediately after insertion of the femoral catheter, bolus 5,000 U of heparin was administered by intra-arterial method, followed by drip infusion of 1,000 U of heparin per 500 ml infusion fluid during the entire period of intervention. After the coiling, heparin was continued intravenously, followed by 100 mg aspirin and 75 mg clopidogrel daily for six months orally or life-long in the case of stent application. Clinical outcome and complication were recorded. Clinic and angiography was followed-up, during the admission and imaging study, such as magnetic resonance angiography (MRA) was scanned six months after the intervention. Clinical outcome was evaluated according to the MRS at 90 days after the initial endovascular procedure.
Statistical analyses
All data were presented as mean ± standard deviation. Neurologic outcome was evaluated with mRS at 90 days after treatment. Comparisons among groups were performed using the unpaired t-test and Fisher exact test. Statistical significance was defined as a probability value 0.05. Statistical package for Windows (SPSS version 19.0, IBM, Armonk, NY, United States) was used for all statistical analyses.
RESULTS
Pre-operative angiographic findings
Of 21 aneurysms, aneurysms were located on the left side in 10 (47.6%) cases and on the right in 11 (52.4%) cases. There was no prevalence in laterality (p > 0.05). The mean size of aneurysm was 13 mm (range 10-25 mm) and the aneurysm size did not show correlation with the successful outcome of the endovascular procedure (p > 0.05). In anatomical configuration of aneurysm, 13 patients showed saccular, seven wide-neck, and one fusiform shape (Table 1).
Clinical and radiologic outcomes
Aneurysm occlusion was performed immediately after embolization with near-complete (Raymond class 1-2) in 20 patients (95.2%) and incomplete (Raymond class 3) in one patient (4.8%). We re-coiled the patient with incomplete occlusion after primary coil of a very large and wide-necked aneurysm; its result was Raymond class 2. Stent assisted coiling was performed in one case, because the operation failed due to thick atherosclerotic plaque. The other patient underwent by-pass surgery (Superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis) initially and then stent assisted coil was attempted (Fig. 1, case No. 6) for the next. Six patients were treated with stent assisted coiling and the other 15 patients were treated by simple coiling (Fig. 2, case No. 2).

Left distal internal carotid artery (ICA) aneurysm (14 mm) with near-complete occlusion. (A, B) Pre-embolization computed tomography (CT) images (A, plain CT; B, 3D-rotational reconstructed CT-angiography) and (C, D) digital subtraction angiogram (DSA) (C, towne view; D, 3D-rotational reconstructed images) demonstrating the 14 mm distal internal carotid artery aneurysm with a wide-neck. (E) Microscopic surgical view of the aneurysm, distal ICA (red arrow) and atherosclerotic aneurysm wall (Black arrow). During (F) and after (G) deployment of a stent assisted coil, (F) the proximal stent guiding tip wire is placed at the middle cerebral artery (MCA) with coil packed coils. And (G) 3D-rotational reconstructed image demonstrates well packed coils and patent parent artery.

Left distal internal carotid artery (ICA) aneurysm (18 mm) with near-complete occlusion. (A, B) Pre-embolization computed tomography (CT) images (A, plain CT; B, 3D-rotational reconstructed CT-angiography) and (C, D) digital subtraction angiogram (DSA) (C, towne view; D, 3D-rotational reconstructed images) demonstrate the 18 mm distal internal carotid artery aneurysm with a wide-neck. (E) Micro-catheter placed in the aneurysm sac. (F, G) DSA after coil deployment. (H) 3D-rotational reconstructed image demonstrates well packed coils.
Fourteen surviving patients underwent clinical and imaging follow-up. Mean follow-up duration was 34.1 months (median 28 months, range 6-102 months). In one patient, six month-follow-up MRA showed major recanalization of the aneurysms, therefore, additional coiling was performed. Clinical outcome of the patients showed correlation with the initial neurologic status (p < 0.05) (Table 2). Overall morbidity and mortality was 19.0%, 33.3% (seven out of 21 patients) and favorable outcome was 47.6% (10 out of 21). In particular, five patients with unruptured aneurysm showed good clinical and angiographic outcomes (Table 2). Delayed thrombotic occlusion occurred in two patients, and resulted in death (Fig. 3, case No. 18). Another five patients died in the hospital, from massive brain edema and/or increased intracranial pressure due to initial subarachnoid hemorrhage, not because of procedure related complication. However, for the relatively larger sized aneurysm, no aneurysm rupture occurred during the endovascular procedure.

Wide-neck right posterior communication artery aneurysm (10 mm) was treated by stent assisted coiling. (A, B) Pre-embolization computed tomography angiography (CTA) (A, plain CT, B, 3D-rotational reconstructed angiography) and (C, D) digital subtraction angiogram (DSA) demonstrating stent assisted coiling. (E, F) Plain CT and 3D-rotational reconstructed CTA after stent assisted coiling show no flow arrest. (G, H) The next day follow-up plain CT and 3D-rotational reconstructed CTA show flow arrest at the right distal ICA and massive brain swelling at the right hemisphere.
DISCUSSION
In ICA aneurysm treatment, the goal is total exclusion of the aneurysm from the cerebral circulation with patent vessel preservation.27)29) Thanks to the advance of microsurgical clipping and endovascular coil embolization technique, management and treatment efficacy of intracranial aneurysms has shown significant improvement.
Large or giant intracranial aneurysms, with complex and refractory lesion, remains as a major challenge to cerebrovascular neurosurgeons.29) These larger or giant aneurysms, most commonly found in the distal ICA segment, where velocity of blood flow is higher, are difficult and have complicated entities requiring a multidisciplinary approach.27)
In patients with neurologic dysfunction and subsequent stroke due to hemodynamic insufficiency as a result of aneurysm in anterior circulation treated by permanent ICA occlusion, symptoms and circulation were improved by external carotid and internal carotid (EC-IC) artery bypass operation. These methods were applied in selected patients with a subgroup having remaining viable brain tissue corresponding to the impaired neurologic function.2) Until now, segmental obliteration after by-pass surgery, STA-MCA has been one of the most acceptable treatments for these complicated aneurysms.10)25)26)27) Many authors have described EC-IC bypass surgery for larger or giant internal carotid artery aneurysms including cavernous, petrous, high cervical, and certain paraclinoid carotid aneurysms that are not amenable to surgical or endovascular treatment.28) Any skilled neurosurgeon can perform difficult ICA aneurysm with bypass surgery. However, the results of definitive multidisciplinary surgical methods remain relatively poor compared to the treatment of smaller aneurysms.8)10)27)
In some rare cases of major artery obliteration (patients who had well tolerated the balloon occlusion test and had sufficient cerebral blood flow), parent artery proximal occlusion (Hunterian's ligation), or ipsilateral internal carotid occlusion is a relatively safe and effective technique that can be used in a significant subset of these patients.13)16)21)26)27)
However, recently advanced radiologic imaging techniques, particularly 3-dimensional image made by computed tomography, magnetic resonance image or angiography, can clearly determine the aneurysm configurations such as vital perforating or distal branch vessels. In general, larger or giant aneurysms are divided according to saccular, wide-neck, and fusiform types in morphology and with or without neck.27)
According to International Subarachnoid Aneurysm Trial (ISAT) and International Study of Unruptured Intracranial Aneurysms (ISUIA), endovascular treatment is relatively more successful in terms of clinical outcome.3)11)14)15)24) However, in results of coiling of large or giant aneurysms, long-term stability of the coil mesh over time was poor, requiring repeated coiling, surgery, and/or parent-vessel balloon occlusion in a large proportion of aneurysms treated primarily with coils.10)20) Recently, according to the development of new endovascular devices, we can treat these large ICA aneurysms with low morbidity and mortality. Stent-assisted coiling is a better alternative than earlier techniques such as parent artery occlusion, which are not always tolerated in the majority of cases.5)21) However, this technique has obvious inherent drawbacks, such as possible damage in the parent artery. Under the technique, overall recanalization rate was achieved in 11.4% of large and giant aneurysms (Raymond class 2), showing higher volumetric occlusion. Long-term follow-up results showed favorable low recanalization rates among terminal bifurcation aneurysms (basilar and ICA terminal).6)
Endovascular treatment of larger or giant intracranial aneurysms is constantly evolving with availability of the latest stent and coil technology. Stent assisted coil treatment of unruptured larger or giant intracranial aneurysms is promising because it appears to be fairly safe and has a preventive effect from rupture.21) During the study of coil packing procedures, stent framework was compressed by packed coils. This event caused major vessel thrombotic occlusion and resulted in a fatal outcome. However, we believe that stent assisted coil treatment represents a considerable alternative to multidisciplinary surgical treatment for larger or giant intracranial aneurysms.
In general, in patients with a clinical symptom of compressive mass effect, coil embolization cannot attenuate the mass effect and symptoms after the operation.5) In one case, mass effect of the left large distal ICA aneurysm caused third nerve palsy. We initially tried a surgical clip, but failed because of severe atherosclerosis of the aneurysmal neck. Stent assisted coiling was performed successfully. Third nerve palsy improved until near complete state after six months from endovascular treatment.
Until now, balloon- and stent-assisted coiling techniques have been one of the milestones in the endovascular treatment of complex intracranial aneurysms. However, flow-diversion devices represent the latest revolution in treatment of endovascular aneurysms. And the paradigm is changing from endosaccular aneurysm embolization to exosaccular parent vessel reconstruction.9) The early results reported in wide-necked large or giant proximal intracranial aneurysms treated using this device have been extremely encouraging.1)4)5)9)12)23)30) However, recently, limitations or drawbacks of the flow-diversion devices, Pipeline embolization device, Balt Extrusion, and SILK flow-diversion device were reported, such as major peri-procedural strokes or aneurysm rupture after treatment. Treatment by endovascular means with flow diversion is one option but is not necessarily the safest or most definitive treatment modality. The ability of perforators to draw blood through the flow-diversion devices remains unproven.9)30)
Recently, advanced imaging techniques can clearly determine the aneurysm configurations. With help of this technique, distal ICA larger aneurysm can be managed with minimal invasive endovascular coiling. Considering the reports of early treatment using a flow-diversion device, proper stent assisted coiling for some large or giant aneurysms is a safer and more effective therapeutic modality, particularly in patients with a closed perforator at the aneurysm or acute angulations or intra-luminal irregularity of the vascular architecture.
CONCLUSION
Using developed intracranial vascular imaging technique and endovascular devices, we can treat complicated distal ICA aneurysms safely and effectively by endovascular technique. In addition, newly developed flow-diversion devices are promising for complicated aneurysms in the distal ICA portion, however, some of these aneurysms still require proper stent assisted coiling rather than flow-diversion devices.
Notes
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.