Hybrid microcatheter angioplasty for refractory cerebral vasospasm
Article information
Abstract
Cerebral vasospasm is a significant cause of morbidity and mortality associated with aneurysmal subarachnoid hemorrhage (aSAH). Intra-arterial chemical and mechanical angioplasty, performed alone or in combination, have been shown to ameliorate cerebral vasospasm and improve patient outcomes. Few options exist for patients who fail these traditional endovascular tactics. We propose a hybrid microcatheter technique that combines the mechanical benefit of transient high pressure induced by microcatheter fluid bolus with a low-dose vasodilator infusion. Five patients with moderate to severe symptomatic vasospasm who failed medical and traditional endovascular management were treated using a hybrid microcatheter technique. All angioplasty procedures were technically successful, and the degree of vasospasm improved following angioplasty. There were no complications related to the cerebral angioplasty procedures. None of the patients required repeat endovascular intervention. Hybrid microcatheter angioplasty may be a useful complement to mechanical or pharmacological techniques in the endovascular management of intractable cerebral vasospasm after aSAH.
INTRODUCTION
Delayed ischemic neurological deficit as a result of cerebral vasospasm is the most common cause of morbidity and mortality following aneurysmal subarachnoid hemorrhage (aSAH) [19]. Cerebral vasospasm occurs in about 60% of all aSAH patients and develops 3-5 days after ictus. The highest risk for development of vasospasm peaks at 5-14 days and resolves over a period of 2-4 weeks [19]. Because vasospasm occurs in a delayed fashion and patients can be non-invasively monitored for clinical and imaging risk factors, it is an important target for aggressive preventative and therapeutic strategies. Myriad medical options are implemented to prevent vasospasm, including calcium channel blockers, endothelial receptor antagonists, statins, and others [5]. Fewer medical options are available to reverse vasospasm if preventive measures are unsuccessful.
Hemodynamic augmentation which includes hypervolemia, hypertension, and hemodilution, is widely used for medical management of clinical and radiographic vasospasm [5]. Known as “triple H therapy”, this technique improves cerebral blood flow through expansion of intravascular blood volume, reduction in blood viscosity, and supportive cerebral blood flow to hypoperfused areas [6]. Triple H therapy improves symptoms in nearly 75% of patients [8], and induced hypertension has been associated with reversal of neurological deficits in approximately two thirds of patients based on observational studies [10,17]. Complications of this strategy include pulmonary edema, hypoxemia, and anasarca, which limit the utility of volume expansion [8]. Literature does not support the institution of the triple-H therapy prior to symptomatic vasospasm [17].
When medical options have been exhausted, or patient comorbidities prevent full application, endovascular treatment should be offered to patients with symptomatic cerebral vasospasm, particularly those who are not rapidly responding to hypertensive therapy (Class IIa; Level of Evidence B) [3]. The primary goal of endovascular treatment is to prevent cerebral infarction by increasing blood flow to territories that have been compromised by vasospasm. The two most common endovascular treatments are mechanical angioplasty (percutaneous transluminal balloon angioplasty) when proximal cerebral vessels are involved and chemical angioplasty, utilizing intra-arterial (IA) bolus of pharmacologic agents when distal arteries are affected [20]. Both techniques may be required in patients with diffuse vasculopathy. Although angiographic outcomes are favorable in nearly 70% of patients [2,3], endovascular therapy has not consistently demonstrated superiority to traditional medical treatment in improving clinical outcomes [20], and there is a risk of procedure-related ischemic complications after endovascular vasospasm therapy [2].
Balloon angioplasty is highly effective in reversing angiographic cerebral vasospasm, but nearly 6% of patients will suffer from complications including vascular injury or perforation, hemorrhage, and even death [1]. Mechanical forces may also cause rupture, and in the case of an improperly purged balloon, air may be introduced into the intracranial circulation [13]. The use of balloon angioplasty is limited to proximal vasculature, because smaller distal arteries prevent mechanical instrumentation at the site of spasm.
Chemical dilatation with IA drug infusion has the advantage of more distal penetration into cortical arterial territories. IA infusions are easier to perform, as they are typically delivered through a catheter positioned in the internal carotid or vertebral arteries [7]. Since the desired effects of these agents include vascular relaxation and vasodilation, patients must be monitored for increased intracranial pressure and hypotension [4,9]. Even when successful, the effects of vasodilator therapy may be transient, and thus require multiple treatments [1,4,20].
To surmount the clinical scenario involving patients who are unable to tolerate IA infusion of vasoactive agents at standard doses due to hypotension [4,20] or whose arterial anatomy limits balloon instrumentation, we offer a hybrid technique that involves microcatheter pressure bolus of dilute calcium-channel blocker or selective phosphodiesterase inhibitor immediately proximal to the site of spasm.
MATERIALS AND METHODS
Once the decision was made to proceed with endovascular therapy, our standard approach was followed: treatment of proximal vasospasm in M1 and accessible M2 segments with balloon angioplasty and IA infusion for distal vasospasm through the diagnostic (or guide) catheter after selective catheterization of the internal carotid artery (ICA) or vertebral artery. After traditional angioplasty approaches have been exhausted and vasospasm in other territories has been addressed, we propose consideration of a novel endovascular technique for refractory proximal vasospasm (Fig. 1). The required endovascular equipment includes a microcatheter of 0.021 to 0.027-inch diameter and a 0.014-inch microwire, secured to a three-way stopcock. A 20 mL syringe with pre-mixed dilute infusate is connected to one port on the stopcock and a 3 mL syringe containing a small volume of the infusate is connected to the other port. The infusate we have used is a 10 mL mixture of 0.125 mg of nicardipine or milrinone in normal sodium chloride solution (0.9% NaCl). More than one bolus was used in most cases, with the maximum infusate we have attempted being 50 mL 0.9% NaCl in 0.625 mg of calcium channel blocker.
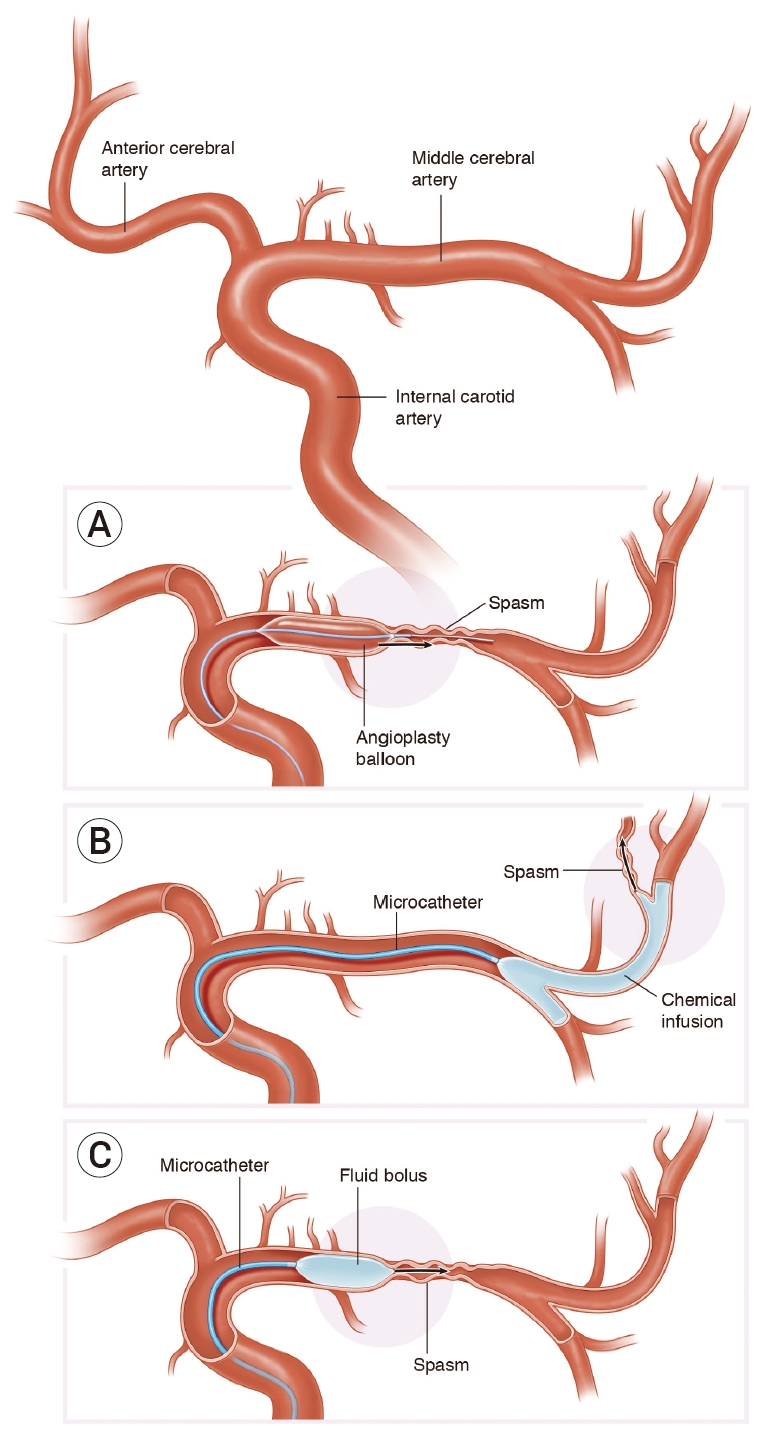
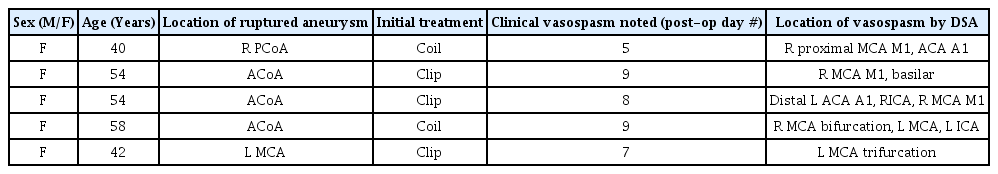
Hybrid microcatheter technique. Traditional endovascular interventions for vasospasm include intra-luminal mechanical disruption using a micro-balloon (A) for proximal cerebral vasospasm, or (B) intra-arterial chemical infusion of to treat distal vasospasm. We propose a hybrid microcatheter technique (C) that combines the mechanical benefit of transient high pressure induced by microcatheter fluid bolus with a low-dose vasodilator infusion.
To perform the technique, a microcatheter and microwire are inserted through the guide catheter (attached to continuous 0.9% NaCl irrigation) and navigated just proximal to the segmental area of vessel narrowing. Once the microcatheter is safely positioned, the wire is removed and the three-way is connected to the microcatheter. After clearing any air from the system, a slow and steady bolus of the infusate is introduced from the 3 mL syringe, refilling, and infusing over two minutes until the 10 mL volume has been consumed. Check flows are then obtained through the guide catheter. The syringes are then refilled, and the process repeated if desired or required. If no changes are seen or results obtained after several attempts, final angiographic runs should be performed and the microcatheter withdrawn.
RESULTS
We present five representative situations (Table 1) where this approach served as a useful adjunct to traditional techniques. After the hybrid technique was performed, the case was completed, and no further treatment was performed on any vessel. Pre-and post-treatment vessel diameters were measured using Centricity PACS (GE Healthcare, Mt Prospect, IL, USA). Percentage change was calculated as the change in value between original and post-treatment diameter divided by the absolute value of the original value, multiplied by 100. We observed a variable but positive response to hybrid microcatheter angioplasty. The average change in vessel diameter across the five vessels treated was 104%, with M1 benefitting more than A1 territories (Table 2). No complications were appreciated angiographically or clinically.
Representative Case 1: Critical vasospasm
An otherwise healthy 40-year-old female presented with aSAH and underwent endovascular coil embolization for a ruptured right posterior communicating artery aneurysm. Post-ictus day 17, transcranial doppler (TCD) studies showed increased velocities in the bilateral middle cerebral arteries (MCA), and she exhibited a new left pronator drift. She was referred for intervention after failed medical therapy. During the procedure, a 3×10 mm HyperGlide balloon (ev3, Plymouth, MN, USA) was introduced over an 0.010-inch X-pedion wire (ev3) into the right anterior cerebral artery (ACA) and ICA terminus (tICA). Serial angioplasty was performed. The microwire would not navigate past the M1 segment of the MCA, and thus balloon angioplasty was not possible. The balloon was replaced with an SL-10 (Stryker Neurovascular, Kalamazoo, MI, USA) over a Synchro 2 microwire (Stryker), and a 0.5 mg bolus of IA nicardipine was delivered at the tICA without effect. The microcatheter was then repositioned proximal to the stenosis in the M1 segment (Fig. 2A), and a bolus of 0.125 mg nicardipine in 10 mL sterile water was firmly pushed into the lumen over a two-minute period (the hybrid microcatheter angioplasty technique). This process was performed three times for a total of 30 mL fluid and 0.375 mg nicardipine. Final angiographic runs demonstrated improvement in vessel caliber of the MCA (Fig. 2B).
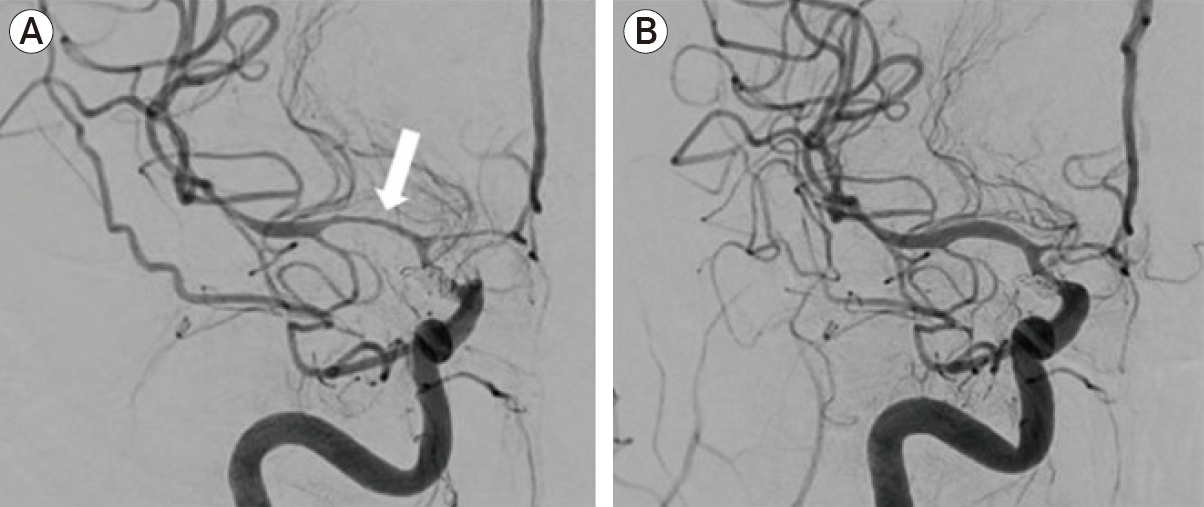
Critical vasospasm. DSA showed severe vasospasm of the right MCA (arrow, A), and ACAs with moderate stenosis of the terminal ICA. Final angiographic runs demonstrated significant improvement in vessel caliber of the MCA (B). DSA, digital subtraction angiography; MCA, middle cerebral artery; ACAs, anterior cerebral arteries; ICA, internal carotid artery.
Representative Case 2: Contiguous intracranial atherosclerotic stenosis (ICAS)
A 54-year-old female with hypertension, ICAS and a remote smoking history underwent emergency clipping of a ruptured anterior communicating artery (ACoA) aneurysm. Six days later, she experienced left lower extremity paresis. CT angiography (CTA) and TCD were consistent with vasospasm in the right MCA, and she underwent digital subtraction angiography (DSA). In addition to diffuse ICAS, distal vasospasm was noted bilaterally, and the patient was treated with IA nicardipine. Three days later (post-ictus day 9) after chemical angioplasty, she was found to have left hemiparesis and radiographic evidence of vasospasm. DSA demonstrated significant stenosis of the right MCA, ACA and tICA and moderate stenosis of the left MCA and tICA. Balloon angioplasty was performed in the left MCA and tICA, and right ACA and tICA with resolution of vasospasm. The right MCA (Fig. 3A) was known to have ICAS at baseline, but comparison to prior DSA showed a contiguous area of ICAS with vasospasm. In order to minimize the risk of plaque rupture and distal embolization, balloon angioplasty was not performed. Instead, an SL-10 microcatheter was navigated over a Synchro 2 microwire and positioned in the mid-M1 segment of the MCA, immediately proximal to the area of presumed ICAS. The hybrid microcatheter angioplasty technique was performed a total of four times, for a total of 40 mL fluid and 0.5 mg nicardipine. Final angiography demonstrated improvement in vessel caliber of the M1 segment but persistence of the known ICAS (Fig. 3B).

Contiguous ICAS. The patient was known to have ICAS at baseline, but comparison to prior DSA showed a contiguous area of ICAS with vasospasm (A). Post-hybrid technique, improvement in MCA caliber is noted without disruption of existing plaque in the MCA (arrow, B). ICAS, intracranial atherosclerotic stenosis; DSA, digital subtraction angiography; MCA, middle cerebral artery.
Representative Case 3: Sharp vessel angle
A 54-year-old female with polycystic kidney disease underwent surgical clipping of a ruptured ACoA aneurysm. Eight days later she demonstrated altered mental status and CTA/TCD consistent with left MCA vasospasm. DSA showed severe spasm in the left ACA (Fig. 4A), which provided supply to both ACA territories. Given the area of stenosis involved a segment of vessel across a 90° angle, balloon inflation in that configuration was considered high risk for vessel rupture. Instead, an SL-10 microcatheter was positioned at the origin of the ACA stenosis, and the hybrid microcatheter angioplasty technique was performed three times, for a total of 30 mL fluid and 0.375 mg nicardipine. Final angiographic runs demonstrated improvement in vessel caliber of the A1 segment (Fig. 4B).
Representative Case 4: Spanning the bifurcation
A 58-year-old female with hypertension underwent coil embolization of a ruptured ACoA. Nine days later, she experienced left hemiparesis and transient cognitive changes. TCD and CTA were consistent with vasospasm in the right MCA, and DSA confirmed severe vasospasm at the right tICA and MCA (with spasm spanning the MCA bifurcation [Fig. 5A]), as well as moderate vasospasm of the left ACA and MCA. Balloon angioplasty was performed in the left ACA, MCA and tICA, and right tICA with good result. Because vasospasm spanned the right MCA bifurcation, balloon angioplasty was not performed. Instead, hybrid microcatheter angioplasty was performed with the microcatheter at the distal M1 segment of the MCA, twice, and then an additional two times in the mid-M1 segment for a total of 40 mL sterile water and 0.5 mg nicardipine. Final angiographic runs demonstrated improvement in vessel caliber of the M1 segment but persistent narrowing of distal vessel caliber (Fig. 5B).

Spanning the bifurcation. DSA confirmed severe vasospasm at the right terminal ICA and MCA with spasm spanning the MCA bifurcation (arrow, A). Final angiographic runs demonstrated improvement in vessel caliber of the M1 segment but persistent narrowing of distal vessel caliber (B). DSA, digital subtraction angiography; ICA, internal carotid artery; MCA, middle cerebral artery.
Representative Case 5: Adjacent unruptured aneurysm
A 42-year-old woman underwent emergent surgical clipping of a ruptured L MCA aneurysm. During subsequent non-invasive brain imaging, she was found to have multiple, smaller unruptured aneurysms. One week after aSAH, she exhibited acute neurological decline with TCD and CTA suggestive of vasospasm. DSA demonstrated vasospasm of the left MCA M1 and M2 segments, along with an unsecured unruptured aneurysm at the MCA trifurcation (Fig. 6A). A 3×10 mm HyperGlide balloon was introduced over an 0.014-inch X-pedion microwire and advanced into the proximal L M1 segment for angioplasty. Post angioplasty angiographic views showed minimal improvement. The balloon was removed and an SL-10 microcatheter was advanced to the area of spasm. The hybrid microcatheter angioplasty technique was performed five times over a period of thirty minutes, for a total IA infusion of 50 mL sterile water and 0.625 mg Milrinone. Post-angiographic views showed improvement in vessel caliber (Fig. 6B).
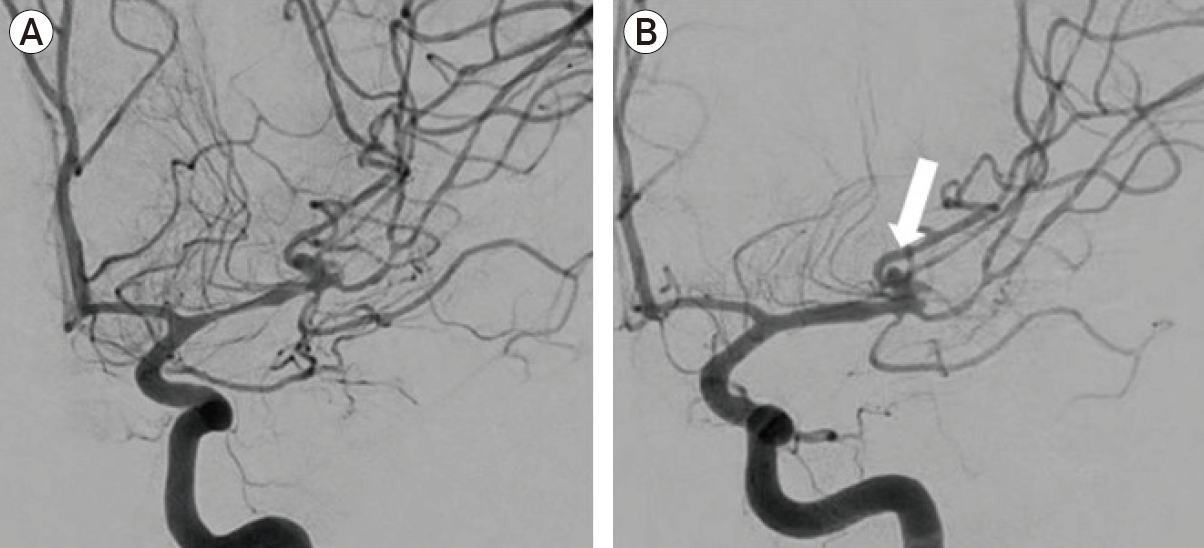
Adjacent aneurysm. DSA demonstrated vasospasm of the left MCA M1 and M2 segments (A), along with an unsecured unruptured aneurysm (arrow, B) at the MCA trifurcation. Post-angiographic views showed improvement in M1 segment vessel caliber (B). DSA, digital subtraction angiography; MCA, middle cerebral artery.
DISCUSSION
Cerebral vasospasm is a major cause of morbidity and mortality in patients who survive aSAH [4,9,11]. When symptomatic vasospasm becomes refractory to maximal medical management, endovascular therapies should be considered. The primary goal of endovascular treatment is to increase cerebral blood flow to prevent cerebral infarction by using inflatable balloons and/or delivery of IA pharmacological vasodilators. For patients who fail traditional endovascular therapy, few options exist. A recent case series proposes stent retrievers as an alternative to angioplasty, given their self-limited and passive expansion of blood vessels [15]. However, these devices require somewhat larger 0.021-inch catheters, are stiff and may cause endothelial disruption [12]. In the absence of other options, we propose a hybrid microcatheter angioplasty technique that combines the mechanical benefit of transient high pressure induced by microcatheter fluid bolus with a low-dose vasodilator infusion.
Several agents have been shown to be safe and effective in the IA treatment of vasospasm after aSAH. A PubMed, Embase and SciFinder database search was performed confirming that at present, the most common drugs for preventing and treating cerebral vasospasm were classified into: calcium channel blockers, magnesium, statins, hormones, phosphodiesterase inhibitors, endothelin-1 antagonists, nitric oxide, heparin and fibrinolytics. Absent guidelines, choice of medication is based on surgeon preference and patient hemodynamic status. Patients who exhibit sensitivity, have contra-indications, or have previously failed therapy with standard agents like nicardipine or verapamil may be treated with milrinone. In patients with a history of tachyarrhythmia or hypokalemia, milrinone should be avoided. The two agents we use routinely for IA management of cerebral vasospasm are milrinone and nicardipine. Milrinone is a phosphodiesterase III inhibitor that affects cyclic adenosine monophosphate pathways with both inotropic and vasodilatory effects. A study investigating the effects of milrinone in 14 patients reported a significant improvement of vasospasm assessed by angiographic control (p<0.0001) [14]. While the specific mechanism is unclear, it has been demonstrated to improve cerebral microcirculation without affecting cardiac output. Nicardipine is a dihydropyridine agent that selectively inhibits calcium ion inflow into the smooth muscle. Due to regional selectivity in cerebrovascular smooth muscle, nicardipine is a potent antihypertensive option for cerebral vasospasm. Nicardipine has been shown to improve TCD velocity studies (p<0.01) and clinical outcomes in 42% of the patients four days post IA treatment, without drug-related complications [7]. Our hybrid technique utilizes a dilute dose, between 10-25% of the suggested amount recommended for distal territory infusion. Our rationale is that the action of the bolus infusion is augmented by the low-dose chemical effect at the site of delivery without causing significant change throughout the cerebral circulation. The ongoing Intra-arterial Vasospasm Trial (iVAST) [16] will provide evidence-based guidance, however several agents (such as milrinone) were not included in the study.
The technical ease of microcatheter navigation allows access to anatomically challenging locations. The composition of the micro-bolus includes sterile water and low dose vasodilators, which minimizes arterial contact and allows for repeated treatments. The transient but relatively high-pressure injection induces a pressure wave across the vasospastic segment that imparts a brief tangential mechanical force on the vessel wall without danger of vessel rupture. This is advantageous over both balloon angioplasty, with its attendant technical risks, and chemical angioplasty, with its lack of mechanical effects on large-vessel vasospasm.
These five representative cases emerged from situations where other modalities were limited by structural issues that prevented our ability to safely introduce equipment to perform angioplasty. In cases of critical vasospasm (case 1), distal wire navigation risks vessel injury and possibly perforation since the 0.010-inch microwire cannot cross the stenosis. In patients where underlying ICAS is contiguous with the vasospastic segment (case 2), balloon dilatation may cause disruption of the atherosclerotic plaque with attendant stroke. When vasospasm abuts a sharp (90 degree) arterial angle (case 3), balloon inflation may stretch or damage the vessel by artificially shaping around the contour of the balloon, such as within the A1 territory, leading to vessel rupture. Vasospasm at the distal M1 segment that extends across the bifurcation (or trifurcation) into the M2 territories may be refractory to IA therapy and inappropriate for balloon angioplasty that stresses the arterial junction (case 4). Finally, there may be unruptured or unsecured aneurysms adjacent to the area of vasospasm (case 5) that increase the risk of perforation and rupture upon wire or balloon navigation. For each of these situations, hybrid microcatheter angioplasty may offer a final therapeutic option.
This report has several limitations, and it is difficult to ascribe quantifiable benefit solely to this technique given the treatments performed to other vessels and segments during the same procedure. We note that all patients in this report are middle-aged females, and results may vary with age or sex of patients. We are unable to assess the duration of dilatory benefit; however, no patient required repeat endovascular therapy or experienced complications. The method we describe has only been attempted in the proximal vasculature (M1 and A1 segments) but may be an option in more distal territories where vasodilator infusion has failed, and balloon angioplasty is not safe or technically feasible. Even using dilute concentrations, the surgeon is reminded to monitor closely for hypotension or changes in intracranial pressure. After hybrid angioplasty, no further mechanical or chemical angioplasty was attempted. Small improvement in vessel caliber from this technique may improve potential for instrumentation and warrant reconsideration for balloon angioplasty.
CONCLUSIONS
In cases of refractory or anatomically inaccessible cerebral vasospasm, the hybrid microcatheter angioplasty technique is a feasible adjunct to traditional mechanical and chemical angioplasty treatment. The direct mechanical hemodynamic effects of injection are minimal, but the focal and rapid force of the IA medication at the site of stenosis has a theoretically higher efficacy than a proximal injection in the neck [18]. More study is needed to understand if the direct transluminal pressure effect provided by this technique is transient or offers more sustained arterial dilation. We welcome collaboration to refine the technique and determine which clinical settings might receive the most benefit.
Acknowledgements
The authors would like to thank Mr. Jonathan Dimes for his illustration of our technique (Fig. 1).
Notes
Disclosure
MW is an educational consultant to Medtronic. MRL receives unrestricted educational grant funding from Medtronic and Stryker, is a consultant for Medtronic, and holds equity interest in Cerebrotech, Synchron and Proprio. The other authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.