 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(2); 2023 > Article |
|
Abstract
Objective
Carotid artery stenting (CAS) is currently widely used for the treatment of carotid artery stenosis. The objective of this study was to analyze the outcomes of CAS performed in a single institution.
Methods
We retrospectively analyzed 313 CAS cases from January 2007 to December 2020, including 206 (66%) symptomatic and 107 (34%) asymptomatic cases. Procedure-related morbidity and mortality were assessed. Rates of periprocedural (≤30 days after CAS) and postprocedural ipsilateral strokes (>30 days after CAS) were also assessed. Logistic regression analysis was used to identify risk factors for the periprocedural complication, in-stent restenosis (ISR), and ipsilateral stroke.
Results
The success rate of CAS was 98%. Among 313 cases, 1 patient died due to hyperperfusion-related intracerebral hemorrhage (ICH). The CAS-related mortality rate was 0.31%. The overall incidence of periprocedural complications is 5.1%. A risk factor for periprocedural complication was a symptomatic carotid artery stenosis (7.3% vs. 0.9%, p=0.016). Twenty cases of ISR occurred during 63.7±42.1 months of follow-up. The overall incidence of ISR was 10.2% (20/196). A risk factors for ISR were diabetes mellitus (17.6% vs. 5.7%, p=0.008) and patients who used Open-cell stents (19.6% vs. 6.9%, p=0.010). The overall incidence of ipsilateral stroke is 5.6%. A risk factors for ipsilateral stroke was ISR (95% CI, p=0.002).
Conclusions
CAS is a safe and effective procedure for carotid artery stenosis. Although the incidence of complications is low, fatal complication such as hyperperfusion- related ICH can occur. To prevent hyperperfusion-related ICH, several methods such as strict blood pressure (BP) control, intentional less widening of stenotic segment should be used. To prevent ISR or stroke occurrence, special attention should be paid to patients who have ISR or ipsilateral stroke risk factors.
Carotid artery angioplasty and stenting (CAS) is now being widely used for the treatment of carotid artery stenosis. Recent studies have shown that CAS is not inferior to carotid endarterectomy (CEA) [4,6]. In the meantime, there have been many studies on the short-term outcome of CAS and studies comparing CEA and CAS, but there are not many studies on the long-term outcome of CAS [3,8]. The purpose of this study was to evaluate the overall outcome and risk factors for periprocedural, postprocedural complications in patients with carotid artery stenosis who underwent CAS in a single institution, and to evaluate the long-term clinical and angiographic outcomes of patients.
Out of total 333 cases, 20 cases were excluded and this retrospective study included a total of 313 CAS procedures between January 2007 and December 2020 (Fig. 1). Except for the follow-up loss cases, the mean follow-up period was 63.7±42.1 months (5.4±3.5 years). Symptomatic patients were defined as those who had a history of cerebral infarction or transient ischemic attack (TIA) symptoms within six months. Symptomatic patients with stenosis of more than 50% according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria and asymptomatic patients with more than 70% stenosis with perfusion defect according to NASCET criteria were included as CAS indications. Patient demographics, procedure details, angiographic and clinical follow-up data were collected. We assessed procedure-related morbidity and mortality. Procedure-related mortality was defined as 30-day mortality. This was defined as death due to complications that occurred within 30 days after CAS. Periprocedural stroke was defined as a stroke that occurred within 30 days after CAS. Postprocedural stroke was defined as stroke that occurred 30 days after CAS. Rates of periprocedural and postprocedural ipsilateral strokes were assessed. Exclusion criteria were as follows: age less than 18 years, simultaneous carotid stenting during mechanical thrombectomy in acute ischemic stroke patients, and carotid stenting during coil embolization. Periprocedural stroke was classified into minor and major according to whether symptoms were temporary or permanent. In-stent restenosis (ISR) was defined as restenosis of more than 50% according to NASCET criteria.
Preoperative imaging studies included carotid ultrasound, carotid computed tomography angiography (CTA), brain and neck magnetic resonance angiography (MRA), perfusion CT or magnetic resonance image (MRI), brain single photon emission computed tomography (SPECT), and digital subtraction angiography.
Dual antiplatelets of aspirin 100 mg and clopidogrel 75 mg were routinely taken for at least 7 days before the procedure. Antiplatelet resistance was assessed with Verify Now assay (Accriva, San Diego, CA, USA). If the preoperative P2Y12 level was 240 or higher, 200 mg of cilostazol was added or clopidogrel was changed to 10 mg of prasugrel. To confirm the risk of myocardial infarction, all patients were routinely evaluated for cardiac risk in consultation with a cardiologist before the procedure.
All interventional procedures were performed by two endovascular neurosurgeons. All procedures were performed under local anesthesia via transfemoral routes. During the procedure, oxygen saturation, electrocardiogram, and blood pressure were monitored.
After the placement of an 8F short arterial introducer sheath on the femoral artery, blood sampling for activated clotting time (ACT) measurement was done. Intravenous bolus injection of 50 IU to 60 IU/kg heparin was done depending on the ACT level in all cases. An 8F soft-tip guiding catheter was placed in the common carotid artery below the carotid stenosis. A distal embolic protection device was employed in all cases.
Preballooning was done in all cases. Before balloon dilatation of stenotic carotid artery, 0.25 mg of atropine was routinely administered. The vital sign was closely monitored. When the pulse rate falls below 50, when the systolic blood pressure falls below 80 mmHg, it was controlled using an atropine or inotropic agent. The selection of the stent was dependent on surgeons’ preference. Except for patients with remaining 50% or more stenosis after stent deployment, post-ballooning was not performed in all patients. After the procedure, patients were admitted to the intensive care unit for one day as a routine. MRI and MRA were taken within 24 hr after CAS. Two cases of hyperperfusion-related intracerebral hemorrhage (ICH) occurred in 2008 and 2009 in our institution. After that, our institution intentionally less widened the stenotic segment, leaving an appropriated amount of residual stenosis to prevent hyperperfusion syndrome.
Patients underwent follow-up carotid CTA six months after the procedure to evaluate ISR. After that, they were followed up by carotid CTA or carotid ultrasound every year. Dual antiplatelet medication was continued for six months after CAS. Clopidogrel was discontinued after six months and aspirin was maintained lifelong.
Statistical analyses were performed using SPSS 26.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean±SD. The significant difference between asymptomatic and symptomatic groups was examined using independent sample t-tests for continuous variables or Chi-square tests for categorical variables. Logistic regression analysis was used to identify risk factors for the periprocedural complication, ISR, and ipsilateral stroke. A p-value of less than 0.05 was considered statistically significant.
Six cases failed due to vascular tortuosity and calcification. A total of 313 cases of carotid artery stenting were performed. The success rate of carotid artery stenting was 98% (307/319). Among them, 206 cases were symptomatic and 107 cases were asymptomatic. Table 1 summarizes baseline characteristics of CAS cases. Low-density lipoprotein (LDL)-cholesterol and total cholesterol levels were significantly higher in the symptomatic group. Left carotid artery lesion was more frequent in the symptomatic group. Most (89.8%) carotid stenosis cases involved carotid bulb. Overall debris capture rate within the filter device during the procedure was 37.9%. Open-cell stents were used in 242 (77.3%) cases. Closed cell stents were used in 71 (22.7%) cases.
Among 313 cases, 1 patient died due to hyperperfusion-related ICH. The CAS-related mortality rate was 0.31%. Periprocedural stroke occurred in 16 (5.1%) cases (1 in asymptomatic and 15 in symptomatic group). Periprocedural stroke rate was significantly higher in the symptomatic group than in the asymptomatic group (7.3% vs. 0.9%, p=0.016) (Table 2). Of 16 cases, 13 had thromboembolic complications, including two major complications. The first case developed middle cerebral artery (MCA) occlusion during the procedure in a patient with severe symptomatic carotid stenosis (95%), which was managed with mechanical thrombectomy. However, neurological sequelae remained. The second case was asymptomatic 81% carotid stenosis. Hemiparesis occurred immediate after CAS. Although cerebral angiography demonstrated no definite occlusion or stenosis of cerebral arteries, left cerebral hemisphere border zone infarction was confirmed on MRI. He had neurological sequelae. Of three cases with hyperperfusion syndrome, two developed hyperperfusion-related ICH on basal ganglia. Of these two patients, one died and the other patient had severe neurological sequelae (modified Rankin Scale grade 5) although craniotomy and hematoma evacuation were performed for both patients. Periprocedural stroke risk was 8.7 times higher in those with symptomatic lesions (Table 3).
Postprocedural complications were largely classified into ISR and occurrence of ipsilateral stroke. Cases with follow-up loss were excluded. Among a total of 196 cases (72 asymptomatic and 124 symptomatic), 20 (10.2%) ISR cases occurred during a mean 63.7±42.1 months follow-up period (15 symptomatic and 5 asymptomatic lesions), including 15 within 3 years and 5 after 3 years. Table 4 summarizes baseline characteristics of ISR occurring in CAS. Those who used closed cell stents, those who had diabetics, and those who had right sided lesions developed ISR more frequently (p<0.05). The rate of ISR was higher in diabetic patients (17.6% vs. 5.7%, p=0.008) and in patients who used closed cell stents (19.6% vs. 6.9%, p=0.010). Logistic regression analysis was performed to identify risk factors for ISR. Diabetic patients were 3.47 times (95% CI, p=0.031) more likely to develop ISR. Patients who used Open-cell stents were 0.33 times (95% CI, p=0.034) less likely to develop ISR than who those who used closed cell stents (Table 5).
Table 6 summarizes the course of treatment for 20 cases of ISR in our institution. Retreatment was planned for 18 cases. Of these 18 cases, two carotid artery occlusions occurred after CAS. One of them was treated with carotid artery bypass surgery and the other was treated with contralateral CAS to augment collateral circulation for contralateral moderate carotid stenosis. Three patients refused retreatment due to cost issues. Of the remaining 13 cases, 9 cases underwent CAS again and 4 cases underwent balloon angioplasty. However, CAS failed in one patient due to carotid artery tortuosity. Of the four cases who underwent balloon angioplasty, two cases had ISR recurrence, 1 showed improvement with the best medical treatment, and 1 had a follow-up loss. In 9 cases who underwent CAS again, ISR recurred in two cases. One patient showed improvement after redo CAS. The other case had a follow-up loss.
Among a total of 195 cases (70 asymptomatic and 125 symptomatic), ipsilateral stroke was identified in 11 cases (5.6%, 10 symptomatic, and 1 asymptomatic). Table 7 shows the results of logistic regression analysis for risk factors of ipsilateral stroke. ISR and total cholesterol level were independent risk factors for ipsilateral stroke during follow-up.
Carotid artery stenting was performed for symptomatic left carotid artery stenosis (NASCET 95%) in a 69-year-old male patient. He was admitted via the emergency room due to right upper extremity motor weakness and dysarthria. MR perfusion showed severe perfusion defect on the left cerebral hemisphere. The procedure was performed using a Precise Stent (Cordis, Santa Clara, CA, USA). Twenty minutes after the procedure, sudden motor dysphasia and worsening of right hemiparesis developed. At that time, his systolic blood pressure had risen to 150 mmHg. Although his blood pressure (BP) dropped immediately to 100 mmHg, hemiparesis and dysphasia aggravated. Brain CT showed ICH on ipsilateral basal ganglia and intraventricular hemorrhage (IVH). Emergency decompressive craniectomy and hematoma removal were performed because his condition deteriorated progressively. It was very difficult to control bleeding from hematoma cavity during surgery because of the antiplatelet and anticoagulant therapy, although heparin was reversed with protamine sulfate immediately after brain CT. After surgery, his neurological status deteriorated. He progressed to brain death and eventually died (Fig. 2).
Carotid artery stenting was performed for the right symptomatic carotid artery stenosis (NASCET 72%) in a 67-year-old male patient. The procedure was performed using a Carotid WALLSTENT (Boston Scientific, USA). Carotid artery occlusion was identified at 12-month follow-up imaging. Extracranial-intracranial bypass surgery was performed to rescue the patient. The patient was discharged home without any complications (Fig. 3).
In this study, the periprocedural stroke rate and periprocedural major stroke rate after CAS were 5.1% and 1.28%, respectively. Periprocedural stroke risk was 8.7 times higher in those with symptomatic lesions. Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) has analyzed CAS and CEA outcomes of 2,502 carotid artery stenosis patients at 117 centers [6]. The CREST trial is the well designed and conducted study among several randomized controlled studies in terms of patient selection, procedure management, and patient follow-up. The incidence of periprocedural stroke was 4.1%. Among them, the rate of major complications was 0.9% [6,21]. In our institution, the stroke rate was higher than that of CREST. It might be because the number of patients was relatively small in our study. In addition, there were many complications in patients treated during the early period with little experience.
In several studies, the rate of visible debris captured in embolic protection device (EPD) varied widely, ranging from 19% to 60% [22,36,41]. This is because the rate changes depending on the stent type used for CAS. Closed-cell stents are known to be effective in confining atheroma to the blood vessel wall, thus reducing the rate of visible debris capture in EPD [41]. For this reason, patients treated with closed-cell stents have a lower risk of intraoperative stroke than patients treated with Open-cell stents [5,9,19]. However, there was no significant difference in stroke incidence according to stent design in our cases.
In our institution, Open-cell stents was used in most (77.3%) cases. The rate of visible debris captured in EPD was 37.9%. Debris capture rate was not significantly different between symptomatic and asymptomatic carotid artery stenosis groups (Table 1).
Reported rates of asymptomatic micro embolism found in postoperative MRI ranged from 14.2% to 22.3% [15,20,22,23,46]. Koklu et al. have retrospectively collected database of patients treated with CAS in a Turkey single center between 2010 and 2020. When a total of 507 patients who underwent CAS were analyzed, postoperative routine MRI showed asymptomatic micro embolism in 72 (14.2%) patients [22]. Gorgulu et al. have collected database for a multicenter randomized prospective study. A total of 279 patients were enrolled, including 139 patients with embolic protection device and 140 patients without embolic protection device [15]. The rate of asymptomatic micro embolism was 22.3% in the patient group who used embolic protection device [15]. Although it was an asymptomatic micro embolism in a weakly functioning brain region, several papers have reported that it can lead to dementia and cognitive disorder during follow-up [14,35]. In our institution, asymptomatic micro emboli were detected by routine postoperative MRI in 37 (11.8%) cases.
Hyperperfusion syndrome is a rare complication that can lead to severe neurological sequelae if it occurs. In our institution, we experienced two (0.64%) cases of hyperperfusion-related ICH, including one mortality.
In the normal brain, when blood pressure changes within an acceptable range, cerebral blood flow is maintained constant by regulating vasomotor tone. However, in patients with longstanding severe carotid stenosis, if the stenotic carotid artery is dilated excessively, cerebral blood vessels do not constrict sufficiently due to vasomotor paralysis, resulting in a sharp increase in blood flow to the brain [12]. For this reason, hyperperfusion syndrome is more likely to occur if the carotid artery stenosis is more than 90%, if the collateral is poor, and if there has been a recent stroke event [1,18,26,34,40]. In our three cases of hyperperfusion syndrome, all were severe symptomatic stenosis with a recent stroke history.
The most important method for the prevention and treatment of hyperperfusion syndrome is strict blood pressure control [29,32,33]. The ideal blood pressure target has not been set. It varies from case to case [29]. Recently, some studies have shown that gradual improvement of cerebral blood flow (CBF) is effective in preventing hyperperfusion syndrome [29,33]. If carotid artery stenosis is severe with many hyperperfusion risk factors, hyperperfusion syndrome can be prevented by intentional underdilatation of stenotic segment, leaving an appropriate amount of residual stenosis. Our institution also used these methods and hyperperfusion-related ICH did not occur since then.
ISR occurred in 10.2% during follow up in our study. ISR is defined as a narrowing of blood vessel diameter by more than 50% compared to baseline at 30 days after the procedure [24]. According to several studies, the occurrence of ISR within 3 years is due to intimal hyperplasia. In the case of ISR occurring after 3 years, recurrent arteriosclerosis is the cause [2,17,44,45]. ISR occurs through two processes: neointimal hyperplasia and vascular remodeling. Neointimal hyperplasia is a process in which the intima thickens due to endothelial cell damage. Vascular remodeling refers to a change in the size of blood vessel involved [42]. Endothelial cell damage during surgical or interventional treatment accelerates neointimal hyperplasia by promoting a cascade of inflammatory mediators. This response can be accelerated by several external factors [31,39,42,47]. Smoking, diabetes, female, hypertension, and stent type are known external factors influencing ISR [44]. In our cases, ISR was higher in diabetic patients (17.6% vs. 5.7%, p=0.008) and in patients who used closed cell stents (19.6% vs. 6.9%, p=0.010). Diabetic patients had 3.47 times (95% CI, p=0.031) more ISR. Patients who used Open-cell stents had 0.33 times (95% CI, p=0.034) less ISR than who used closed cell stents (Table 5). In diabetic patients, hyperglycemia and dysregulated matrix protein production can induce reactive oxygen species. This accelerates atherosclerosis and hyperlipidemia, which in turn promotes ISR [47]. Because the closed cell stent is rigid, it can induce straightening of the carotid artery and stimulate neointimal hyperplasia and ISR by causing vessel wall stress [27]. This theory is supported by the fact that the rate of occurrence of in stent restenosis is high within 3 years [48]. In our case, as in previous studies, more ISR occurred within 3 years. Of a total of 20 cases of ISR, 15 cases occurred within 3 years. Only 5 cases occurred after 3 years. Among 20 ISR cases, 14 patients underwent retreatment, including 8 redo CAS, 4 balloon angioplasty, 1 contralateral CAS due to carotid artery occlusion, and 1 bypass surgery (Table 6).
There are no treatment guidelines or definitive consensus for ISR. Therefore, treatment of ISR should be decided by the physician in consideration of various factors such as clinical symptoms, collateral circulation, decrease in perfusion, and degree of stenosis. Treatment for ISR can be classified into best medical treatment, endovascular procedure, and surgery. Although the best medical treatment is effective for most patients, one report has shown that more aggressive treatment is required because it cannot reliably prevent stroke [38]. Although there have been no randomized controlled trials evaluating whether symptomatic restenosis should be treated medically or surgically, the latest European Society for Vascular Surgeons (ESVS) guidelines suggest that the same criteria used for symptomatic carotid artery stenosis patients be adopted [10]. Based on this, symptomatic restenosis (more than 50%) patients should be treated by CAS or CEA within 14 days of symptoms. In the case of asymptomatic ISR patients, there is no clear guideline with a lot of debate. Thus, it is necessary to decide whether to just follow-up, preform the best medical treatment, provide an interventional treatment, or give a surgical treatment in consideration of various factors mentioned earlier.
When it is decided to perform revascularization in a patient with ISR, we should decide whether to perform a surgery or an endovascular treatment. According to recent studies, in the case of post-CEA ISR, there is a risk of complication related to revision CEA due to difficulties such as scar formation [13,16]. Therefore, in case of post-CEA ISR, carotid artery stenting is recommended [13,16]. Balloon angioplasty can also be considered. Although balloon angioplasty is inferior to CAS in terms of short duration and so on, there are reports that it shows satisfactory results in the treatment of ISR [7,25,37]. Carotid bypass operation can also be performed for treatment of ISR. Stiolo et al. have retrospectively collected database on patients treated with carotid bypass in two high-volume Italian centers between 2008 and 2016 for symptomatic high-grade ISR after CAS. According to this database, only one (7.6%) patient had a transient cranial nerve damage after carotid bypass. Except for this case, other patients were discharged without any complications. A 100% patency rate was confirmed at a mean follow-up of 41.2±18.2 months [43].
As in several studies, balloon angioplasty was inferior to CAS in terms of recurrence rate [11,28,30]. ISR recurred in 2 out of 4 cases in balloon angioplasty cases. ISR occurred in 2 out of 8 cases in redo CAS cases (50% vs. 25%). During follow-up, two patients with carotid artery occlusion were identified. One patient showed improvement of blood flow after undergoing contralateral CAS. One patient showed improved blood flow by performing carotid bypass using saphenous vein. Demonstration case 2 is a patient who underwent carotid bypass operation due to carotid artery occlusion. Other ISR patients were treated using various treatment options. Except for treatment refusal cases, ISR patients were discharged without any problems. In this way, when a problem occurred after CAS, our institution was able to treat patients more effectively by selecting a treatment method considering various factors based on various treatment options.
Among a total of 195 cases (70 asymptomatic, 125 symptomatic), ipsilateral stroke occurred in 11 cases (10 symptomatic and one asymptomatic). The incidence was 5.6%. In several studies, 5-year cumulative risk and percentage were calculated to analyze postprocedural stroke. The 5-year cumulative risk of fetal stroke was less than 7% in most studies [4,6]. International Carotid Stenting Study (ICSS) has compared outcomes of a total 1,713 carotid artery stenosis patients with a median follow-up of 4.2 years. The 5-year cumulative fatal or disabling stroke risk was 6.4% in ICSS and that of any stroke was 15.2% [4]. The CREST study showed similar results [6]. In our institution, a significant 5-year cumulative risk of postprocedural stroke could not be obtained due to insufficient number of cases. However, during a median follow-up of 5.3 years, the incidence of postprocedural stroke was 5.6%, which was low, similar to other studies [4,6]. Other than that, no other problems occurred.
CAS is a safe and effective procedure for carotid artery stenosis. Although its complication rate is low, fatal complication of hyperperfusion-related ICH can occur. To prevent this complication, strict BP control is mandatory. Furthermore, ISR can occur in 10% of CAS during long-term follow up. Special attention should be paid to patients with diabetes, symptomatic carotid stenosis, and patients treated with closed cell stents who have a high risk of ISR after CAS.
Fig. 1.
Patients flow chart. Inclusion criteria were as follows: Symptomatic patients with carotid stenosis of more than 50% according to NASCET criteria and asymptomatic patients with more than 70% carotid stenosis with perfusion defect. Exclusion criteria were as follows: age less than 18 years, simultaneous carotid stenting during mechanical thrombectomy in acute ischemic stroke patients, and carotid stenting during coil embolization. CAS, carotid artery stenting; NASCET, North American Symptomatic Carotid Endarterectomy Trial
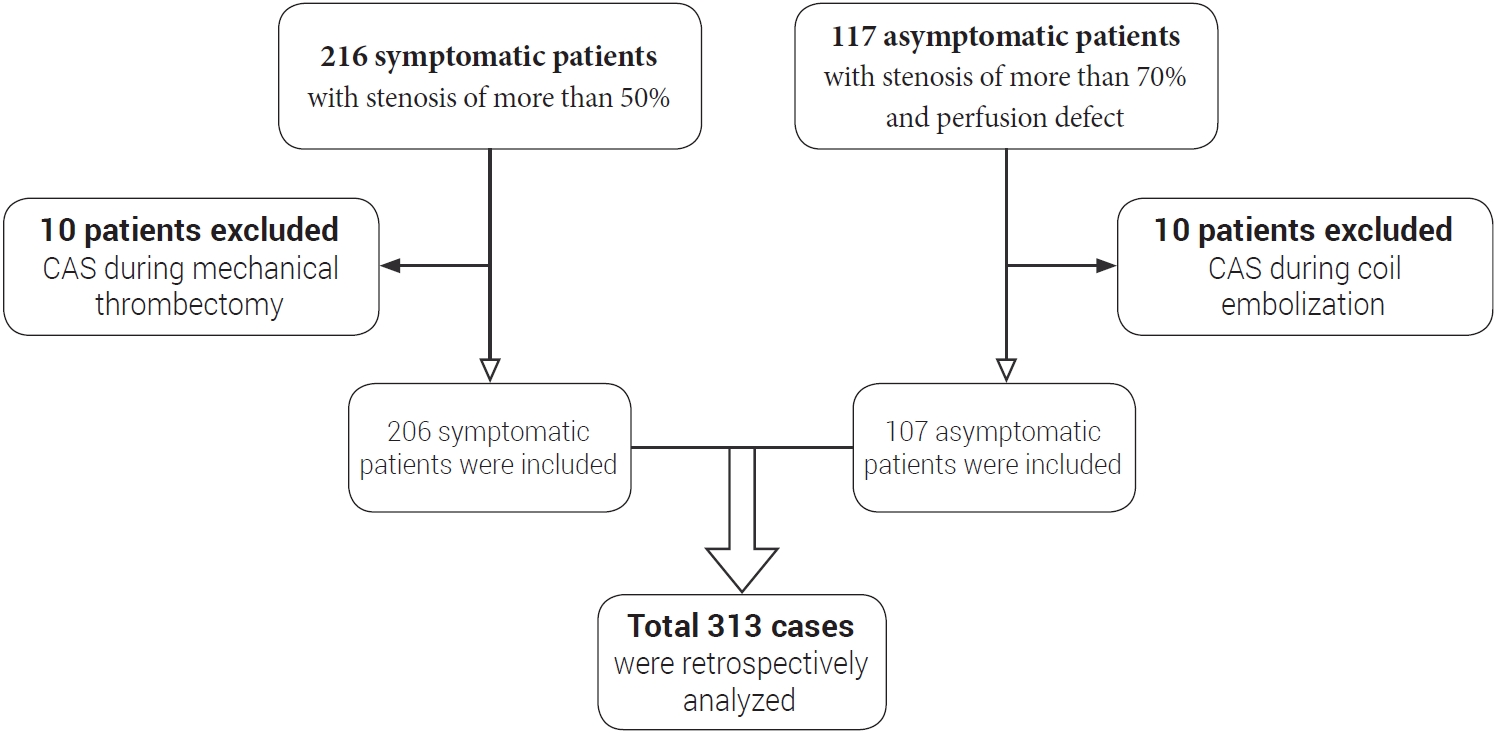
Fig. 2.
Digital subtraction angiography and brain CT image of demonstrative case 1 showing hyperperfusion-related ICH on left basal ganglia. (A) Preoperative digital subtraction angiography showing NASCET 95% stenosis, (B) Postoperative digital subtraction angiography showing residual stenosis about 30% using NASCET criteria, (C) Brain CT image at 20 minutes after procedure showing hyperperfusion-related ICH on left basal ganglia and intraventricular hemorrhage, (D) Postoperative brain CT image showing craniectomy and catheter insertion state. CT, computed tomography; ICH, intracerebral hemorrhage; NASCET, North American Symptomatic Carotid Endarterectomy Trial
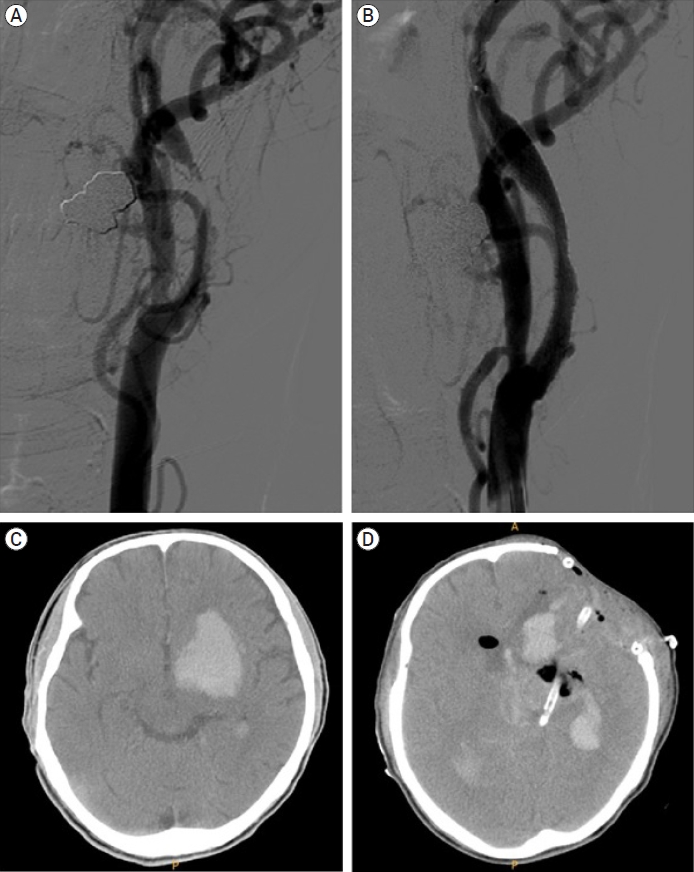
Fig. 3.
Demonstrative case 2 showing ISR retreatment. (A) Preoperative digital subtraction angiography showing NASCET 72% stenosis, (B) Postoperative digital subtraction angiography showing residual stenosis about 0% using NASCET criteria, (C) Digital subtraction angiography at 18 months after CAS showing complete occlusion of carotid artery, (D) MRA image performed at 24 hours after carotid bypass operation showing successful blood flow, (E) Time to peak image conducted at 18 month after CAS showing time to peak elongation, demonstrating perfusion decrease, (F) Time to peak image conducted at 24 hours after carotid bypass operation showing perfusion improvement. ISR, in-stent restenosis; NASCET, North American Symptomatic Carotid Endarterectomy Trial; CAS, carotid artery stenting; MRA, magnetic resonance angiography
Table 1.
Baseline characteristics of carotid artery stenting cases
| Variable | Total (n=313) (%) | Asymptomatic group (n=107) (%) | Symptomatic group (n=206) (%) | p-value |
|---|---|---|---|---|
| Age, mean±SD (years) | 69.4±9.3 | 70.5±8.3 | 68.8±9.7 | 0.126 |
| Female | 53 (16.9) | 17 (15.9) | 36 (17.5) | 0.772 |
| Hypertension | 239 (76.4) | 85 (79.4) | 154 (74.8) | 0.355 |
| Diabetes mellitus | 124 (39.6) | 42 (39.3) | 82 (39.8) | 0.924 |
| Smoking | 98 (31.3) | 28 (26.2) | 70 (34.0) | 0.157 |
| LDL cholesterol, mean±SD (mg/dL) | 97.6±38.6 | 88.2±37.0 | 102.4±38.6 | 0.002 |
| Total cholesterol, mean±SD (mg/dL) | 166.8±44.2 | 157.3±42.5 | 171.7±44.4 | 0.006 |
| Side | ||||
| Left | 157 (50.2) | 43 (40.2) | 114 (55.3) | 0.011 |
| Right | 156 (49.8) | 64 (59.8) | 92 (44.7) | |
| Blub lesion | 281 (89.8) | 96 (89.7) | 185 (89.8) | 0.981 |
| Stenosis length, mean±SD (mm) | 17.1±10.1 | 17.5±10.6 | 16.9±9.9 | 0.599 |
| Previous stroke history | 117 (37.4) | 38 (35.5) | 79 (38.3) | 0.623 |
| Pre-procedural NASCET, mean±SD (%) | 80.3±13.1 | 79.8±11.4 | 80.6±14.0 | 0.628 |
| Post-procedural NASCET, mean±SD (%) | 20.6±13.3 | 23.9±13.4 | 18.9±12.9 | 0.002 |
| Filter debris capture | 80 (37.9) | 23 (32.9) | 57 (40.4) | 0.286 |
| Stent type used | ||||
| Open cell* | 242 (77.3) | 77 (72.0) | 165 (80.1) | 0.103 |
| Closed cell** | 71 (22.7) | 30 (28.0) | 41 (19.9) |
Table 2.
Periprocedural stroke and CAS-related mortality
Table 3.
Risk factors for peri-procedural complications in patients with carotid artery stenting (16/313)
Table 4.
Baseline characteristics of in-stent restenosis (ISR)
| Variable | Total (n=196) (%) | No in-stent restenosis (n=176) (%) | In-stent restenosis (n=20) (%) | p-value |
|---|---|---|---|---|
| Age, mean±SD (years) | 67.1±9.3 | 67.1±9.4 | 67.9±9.4 | 0.718 |
| Follow up period, mean±SD (months) | 63.7±42.1 | 62.6±42.3 | 72.8±41.0 | 0.308 |
| Female | 34 (17.3) | 31 (17.6) | 3 (15.0) | 0.999 |
| Hypertension | 150 (76.5) | 133 (75.6) | 17 (85.0) | 0.418 |
| Diabetes mellitus | 74 (37.8) | 61 (34.7) | 13 (65.0) | 0.013 |
| Smoking | 65 (33.2) | 56 (31.8) | 9 (45.0) | 0.235 |
| LDL cholesterol, mean±SD (mg/dL) | 96.1±36.2 | 96.2±36.9 | 95.7±29.3 | 0.954 |
| Total cholesterol, mean±SD (mg/dL) | 167.9±43.4 | 168.9±43.8 | 158.9±39.7 | 0.328 |
| Side | ||||
| Left | 97 (49.5) | 92 (52.3) | 5 (25.0) | 0.021 |
| Right | 99 (50.5) | 84 (47.7) | 15 (75.0) | |
| Blub lesion | 175 (89.3) | 155 (88.1) | 20 (100.0) | 0.137 |
| Stenosis length, mean±SD (mm) | 16.4±9.3 | 16.1±9.1 | 19.3±10.8 | 0.135 |
| Previous stroke history | 70 (35.7) | 64 (36.4) | 6 (30.0) | 0.574 |
| Pre-procedural NASCET, mean±SD (%) | 80.0±13.6 | 79.8±13.7 | 81.9±13.1 | 0.517 |
| Post-procedural NASCET, mean±SD (%) | 20.0±13.3 | 19.9±12.8 | 20.6±17.2 | 0.881 |
| Stent type used | ||||
| Open cell* | 145 (74.0) | 135 (76.7) | 10 (50.0) | 0.01 |
| Closed cell** | 51 (26.0) | 41 (23.3) | 10 (50.0) |
Table 5.
Risk factors for in-stent restenosis (ISR) in patients with carotid artery stenting (20/196)
Table 6.
Course of in-stent restenosis (ISR) retreatment
Table 7.
Risk factors for ipsilateral stroke in patients with carotid artery stenting (11/195)
REFERENCES
1. Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol. 2004 May;43(9):1596-601.

2. AbuRahma AF, Abu-Halimah S, Hass SM, Nanjundappa A, Stone PA, Mousa A, et al. Carotid artery stenting outcomes are equivalent to carotid endarterectomy outcomes for patients with post-carotid endarterectomy stenosis. J Vasc Surg. 2010 Nov;52(5):1180-7.


3. AbuRahma AF, AbuRahma ZT, Santini A, Beasley M, Davis M, Lee A, et al. A single-center experience of 30-day perioperative and one year clinical outcomes of transcarotid artery revascularization in 100 consecutive patients. Vascular. 2022 May;17085381221106330.


4. Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015 Feb;385(9967):529-38.



5. Bosiers M, de Donato G, Deloose K, Verbist J, Peeters P, Castriota F, et al. Does free cell area influence the outcome in carotid artery stenting? Eur J Vasc Endovasc Surg. 2007 Feb;33(2):135-41; discussion 42-3.


6. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016 Mar;374(11):1021-31.



7. Chakhtoura EY, Hobson RW 2nd, Goldstein J, Simonian GT, Lal BK, Haser PB, et al. In-stent restenosis after carotid angioplasty-stenting: incidence and management. J Vasc Surg. 2001 Feb;33(2):220-5; discussion 225-6.


8. Diyora B, Chheda RM, Dhall G, Gupta P, Dewani K, Mulla M, et al. Carotid endarterectomy and carotid artery stenting for symptomatic carotid stenosis: an experience of a hybrid neurosurgeon in a developing nation. Neurol India. 2022 JanFeb;70(1):94-101.


9. Doig D, Turner EL, Dobson J, Featherstone RL, Lo RT, Gaines PA, et al. Predictors of stroke, myocardial infarction or death within 30 days of carotid artery stenting: results from the international carotid stenting study. Eur J Vasc Endovasc Surg. 2016 Mar;51(3):327-34.



10. Eckstein HH. European society for vascular surgery guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur J Vasc Endovasc Surg. 2018 Jan;55(1):1-2.


11. Fanelli F, Boatta E, Cannavale A, Corona M, Lucatelli P, Wlderk A, et al. Carotid artery stenting: analysis of a 12-year singlecenter experience. J Endovasc Ther. 2012 Dec;19(6):749-56.


12. Farooq MU, Goshgarian C, Min J, Gorelick PB. Pathophysiology and management of reperfusion injury and hyperperfusion syndrome after carotid endarterectomy and carotid artery stenting. Exp Transl Stroke Med. 2016 Sep;8(1):7.




13. Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Jan;42(1):227-76.


14. Gensicke H, van der Worp HB, Nederkoorn PJ, Macdonald S, Gaines PA, van der Lugt A, et al. Ischemic brain lesions after carotid artery stenting increase future cerebrovascular risk. J Am Coll Cardiol. 2015 Feb;65(6):521-9.



15. Gorgulu S, Sahin M, Norgaz NT, Pala S, Sari M, Yalcin AA, et al. Carotid artery stenting without embolic protection: A randomized multicenter trial (the CASWEP trial). Interv Neuroradiol. 2022 Apr;15910199221094388.


16. Hobson RW 2nd, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008 Aug;48(2):480-6.


17. Hunter GC. Edgar J. Poth Memorial/W.L. Gore and Associates, Inc. Lectureship. The clinical and pathological spectrum of recurrent carotid stenosis. Am J Surg. 1997 Dec;174(6):583-8.

18. Jansen C, Sprengers AM, Moll FL, Vermeulen FE, Hamerlijnck RP, van Gijn J, et al. Prediction of intracerebral haemorrhage after carotid endarterectomy by clinical criteria and intraoperative transcranial Doppler monitoring. Eur J Vasc Surg. 1994 May;8(3):303-8.


19. Jansen O, Fiehler J, Hartmann M, Bruckmann H. Protection or nonprotection in carotid stent angioplasty: the influence of interventional techniques on outcome data from the SPACE Trial. Stroke. 2009 Mar;40(3):841-6.


20. Kashiwazaki D, Kuwayama N, Akioka N, Noguchi K, Kuroda S. Carotid plaque with expansive arterial remodeling is a risk factor for ischemic complication following carotid artery stenting. Acta Neurochir (Wien). 2017 Jul;159(7):1299-304.



21. Kim NY, Choi JW, Whang K, Cho SM, Koo YM, Kim JY. Neurologic complications in patients with carotid artery stenting. J Cerebrovasc Endovasc Neurosurg. 2019 Jun;21(2):86-93.




22. Koklu E, Arslan S, Sarionder Gencer E, Bayar N, Avci R, Ozgunoglu EC. Six-year outcomes of carotid artery stenting performed with multidisciplinary management in a single center. Anatol J Cardiol. 2021 Jun;25(6):385-94.


23. Koklu E, Arslan S, Sarionder Gencer E, Bayar N, Ureyen CM, Erkal Z, et al. Asymptomatic cerebral emboli following carotid artery stenting: a diffusion-weighted MRI study. Anatol J Cardiol. 2022 Apr;26(4):298-304.



24. Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. 2012 Sep;11(9):755-63.



25. Lal BK, Hobson RW 2nd, Goldstein J, Geohagan M, Chakhtoura E, Pappas PJ, et al. In-stent recurrent stenosis after carotid artery stenting: life table analysis and clinical relevance. J Vasc Surg. 2003 Dec;38(6):11162-8; discussion 1169.

26. Macfarlane R, Moskowitz MA, Sakas DE, Tasdemiroglu E, Wei EP, Kontos HA. The role of neuroeffector mechanisms in cerebral hyperperfusion syndromes. J Neurosurg. 1991 Dec;75(6):845-55.


27. Megaly M, Alani F, Cheng CI, Ragina N. Risk factors for the development of carotid artery in-stent restenosis: multivariable analysis. Cardiovasc Revasc Med. 2021 Mar;24:65-9.


28. Miyachi S. Carotid angioplasty and stenting for occlusive diseases. Adv Tech Stand Neurosurg. 2022 44:209-23.


29. Mo D, Luo G, Wang B, Ma N, Gao F, Sun X, et al. Staged carotid artery angioplasty and stenting for patients with high-grade carotid stenosis with high risk of developing hyperperfusion injury: a retrospective analysis of 44 cases. Stroke Vasc Neurol. 2016 Dec;1(4):147-53.



30. Montorsi P, Galli S, Teruzzi G, Troiano S, Caputi L, Gili S, et al. Case report: a cluster of complications during carotid artery stenting managed with peripheral, coronary, and imaging techniques. Front Cardiovasc Med. 2021 Sep;8:712963.



31. Moore WS, Kempczinski RF, Nelson JJ, Toole JF. Recurrent carotid stenosis: results of the asymptomatic carotid atherosclerosis study. Stroke. 1998 Oct;29(10):2018-25.


32. Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg. 2007 Dec;107(6):1130-6.


33. Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, et al. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg. 2003 Sep;99(3):504-10.


34. Ouriel K, Shortell CK, Illig KA, Greenberg RK, Green RM. Intracerebral hemorrhage after carotid endarterectomy: incidence, contribution to neurologic morbidity, and predictive factors. J Vasc Surg. 1999 Jan;29(1):82-7; discussion 87-9.


35. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009 Nov;8(11):1006-18.


36. Reimers B, Corvaja N, Moshiri S, Sacca S, Albiero R, Di Mario C, et al. Cerebral protection with filter devices during carotid artery stenting. Circulation. 2001 Jul;104(1):12-5.


37. Setacci C, de Donato G, Setacci F, Pieraccini M, Cappelli A, Trovato RA, et al. In-stent restenosis after carotid angioplasty and stenting: a challenge for the vascular surgeon. Eur J Vasc Endovasc Surg. 2005 Jun;29(6):601-7.


38. Shahidi S, Owen-Falkenberg A, Gottschalksen B, Ellemann K. Risk of early recurrent stroke in symptomatic carotid stenosis after best medical therapy and before endarterectomy. Int J Stroke. 2016 Jan;11(1):41-51.



39. Simonetti G, Gandini R, Versaci F, Pampana E, Fabiano S, Stefanini M, et al. Carotid artery stenting: findings based on 8 years’ experience. Radiol Med. 2009 Feb;114(1):95-110.



40. Son S, Choi DS, Kim SK, Kang H, Park KJ, Choi NC, et al. Carotid artery stenting in patients with near occlusion: a single-center experience and comparison with recent studies. Clin Neurol Neurosurg. 2013 Oct;115(10):1976-81.


41. Sprouse LR 2nd, Peeters P, Bosiers M. . The capture of visible debris by distal cerebral protection filters during carotid artery stenting: Is it predictable? J Vasc Surg. 2005 Jun;41(6):950-5.


42. Stilo F, Montelione N, Calandrelli R, Distefano M, Spinelli F, Di Lazzaro V, et al. The management of carotid restenosis: a comprehensive review. Ann Transl Med. 2020 Oct;8(19):1272.



43. Stilo F, Sirignano P, Montelione N, Mansour W, Capoccia L, Catanese V, et al. Bypass for symptomatic in-stent carotid restenosis. Int J Cardiol. 2017 Dec;249:392-5.


44. Texakalidis P, Tzoumas A, Giannopoulos S, Jonnalagadda AK, Jabbour P, Rangel-Castilla L, et al. Risk factors for restenosis after carotid revascularization: a meta-analysis of hazard ratios. World Neurosurg. 2019 May;125:414-24.


45. Trisal V, Paulson T, Hans SS, Mittal V. Carotid artery restenosis: an ongoing disease process. Am Surg. 2002 Mar;68(3):275-9; discussion 279-80.



46. Tsai CH, Chen YH, Lin MS, Huang CC, Hung CS, Yeh CF, et al. The periprocedural and 30-day outcomes of carotid stenting in patients with carotid artery near-occlusion. Sci Rep. 2021 Nov;11(1):21876.




- TOOLS
-
METRICS

-
- 2 Crossref
- 0 Scopus
- 1,940 View
- 84 Download
- ORCID iDs
-
Seok Mann Yoon

https://orcid.org/0000-0002-0048-6309 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



