 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(3); 2023 > Article |
|
Abstract
Objective
Microvascular anastomosis, particularly side-to-side (STS) bypass, is a complex surgical procedure. While several suture techniques exist, none of them is superior to the others. We assessed the association between various STS bypass techniques and vessel twisting using chicken wing training models.
Methods
Three suture techniques were compared over an anterior wall suture procedure. The unidirectional continuous suture (UCS) group used a downward “right-to-left” continuous suture. The reverse continuous suture (RCS) group used a downward “left-to-right” continuous suture. The interrupted suture (IS) group used the standard interrupted suture. The number of samples in each of the three groups was 30 (n=90). We compared the incidence of vessel twisting and rotation angles across groups.
Results
Vessel twisting occurred in 96.7%, 56.7%, and 0% of the cases in the UCS, IS, and RCS groups, respectively. The incidence of vessel twisting differed significantly in all 3 groups (p<0.001), with an apparent trend (p=0.002). The mean rotation angles were 201˚±90.6˚, 102˚±107.6˚, and 0˚ in the UCS, IS, and RCS groups, respectively, which were significantly different (p<0.001). On excluding cases without twisting, the rotation angles of twisted vessels in the UCS and IS groups were 207.9˚±83.7˚ and 180˚±77.9˚, respectively, which yielded a significant difference between these groups (p<0.001).
Cerebrovascular bypass surgery remains a popular treatment option for complex cerebral vascular diseases despite recent advances in endovascular treatment [1,5,8,9,13,20,21,26]. Owing to the complexity and inconsistent performance of the intracranial microsurgical bypass procedures, particularly the side-to-side (STS) technique, meticulous training is essential for better outcomes. The training model using chicken wing arteries is well-known [7,15].
A neurovascular surgeon (DHJ, right-handed junior neurosurgeon), an author of this article, noticed differences in the occurrence of structural changes in chicken wing artery STS training between continuous and interrupted suture groups. The structural change was a counterclockwise twisting of vessels after the STS bypass, which occurred more frequently in the continuous suture group. Based on this observation, the authors hypothesized that the incidence of vessel twisting would differ depending on the suture technique used. This study aimed to assess the association between the STS technique used and vessel twisting in chicken wing training models.
From July 1, 2020, to April 28, 2021, we dissected brachial arteries from chicken wings sold in refrigerated packaging in a local market. We prepared the arteries using a microscope (SZM-45B2, Madell Technology Corporation, Ontario, CA, USA). A total of 90 dissected arteries were prepared and then randomly divided into 3 groups of 30 arteries each. The dissected arteries were cut in half, and the diameter of the cut site was measured using a microscope. The bypass proceeded with 2 divided arteries.
The arteriotomy was performed using the standard procedure at a ratio of 3:1 to the artery diameter. An Ethilon W2860 10-0 polyamide suture (Ethicon, Somerville, NJ, USA) and an SZM-45B2 microscope (Madell Technology Corporation, Ontario, CA, USA) were used in all 3 groups. In all groups, the posterior wall sutures were performed using the same method. After completing the lower stay suture, the upper stay suture was performed using a new thread. Subsequently, the posterior wall suture was performed with a “right-to-left” downward-directed continuous suture with a long tail of the upper stay suture thread.
In the continuous suture groups, the anterior wall suture was performed using the new thread, which started immediately below the upper stay suture. The suture proceeded downward for better visualization and to secure the suturing field [5]. In the unidirectional continuous suture (UCS) group, a downward continuous suture was performed in the “right-to-left” direction. In the reverse continuous suture (RCS) group, a downward continuous suture proceeded in the “left-to-right” direction. A usual interrupted suture was performed for the anterior wall suture in the interrupted suture (IS) group.
Vessel twisting is defined as vessel rotation after bypass procedure that renders the anterior suture wall partially invisible (Fig. 1). The rotation angle was measured based on the lower stay suture knot position in the plane perpendicular to the longitudinal axis of the vessels (Fig. 2). The 12 o’clock direction was counted as 0 degree and the angle was measured counterclockwise. After measuring the rotation angle, all specimens were incised and a single wall suture was confirmed.
We used the SPSS (IBM SPSS Statistics Standard, IBM, Chicago, IL, USA) and R Studio (R 4.0.3, R Studio, Boston, MA, USA) programs for statistical analysis. The Kruskal- Wallis test was run on SPSS to assess the differences in artery sizes and rotation angles among the 3 groups. We implemented linear-by-linear association with SPSS to analyze the incidence of twisting by groups. Furthermore, the Cochrane-Armitage trend test was used to assess incidence on R Studio. Values are expressed as means±standard deviations. A p-value of less than 0.05 was considered significant.
The total diameter of the 90 arteries ranged from 0.7 to 2.1 mm (1.06±0.18 mm). The diameters in the UCS, IS, and RCS groups were 0.8-1.3 mm (1.03±0.13 mm), 0.7-1.4 mm (1.06±0.11 mm), and 0.7-2.1 mm (1.09±0.15 mm), respectively. The arterial caliber did not significantly differ among the 3 groups (p=0.64).
Vessel twisting occurred in 96.7% (29/30) of cases in the UCS group, 56.7% (17/30) in the IS group, and 0% (0/30) in the RCS group, and the twist was counterclockwise in all these cases. The incidence of vessel twisting differed significantly among groups (p<0.001). We observed a trend in the incidence of vessel twisting (p=0.002), and the order was the highest in the UCS group, followed by that in the IS group and the RCS group (Table 1). The rotation angles from all samples were 201˚±90.6˚, 102˚±107.6˚, and 0˚ in the UCS, IS, and RCS groups, respectively. The difference was statistically significant among the 3 groups (p<0.001). In the post hoc tests, 2 groups were compared for rotation angles, which showed a significant difference (p<0.001). The rotation angle of the twisted samples was 207.9˚±83.7˚ in the UCS group and 180˚±77.9˚ in the IS group; the difference was significant (p<0.001).
Although several methods for microsurgical STS bypass exist [26], no method has been proven to be superior over the other. Most studies on cerebrovascular bypass procedures are retrospective studies, making comparisons challenging [1,8,9,13,16,18-21]. Most studies on microsurgical bypass not confined to cerebrovascular bypass have considered the extent of bleeding, procedure time, complications, and differences in suture techniques [4,12,14,17,23,24]. Only few comparative experimental studies that are not clinical studies on bypass techniques exist. Hafez et al. [6] and Bot et al. [3] published experimental studies comparing suture techniques affecting the quality of end-to-side (ETS) and end-to-end (ETE) anastomosis, respectively. Topalan et al. [24] and Levi et al. [14] studied the effects of preoperatively-created vessel torsion on anastomosis patency in ETE and ETS bypass, respectively, using a rat model. Unlike previous studies on preoperative conditions, our study focused on vessel twisting as a result of an STS bypass, which has not been studied before.
Structurally, the continuous suture generates an oblique reaction force along the suture surface. If the suture specimen is sufficiently large and the suture bites are adequately small, the reactive force will not have a significant effect on the suture site. However, structural changes may occur in microsurgery. Occurrence of corneal rotation during keratoplasty using continuous sutures is well documented [2,25]. Keratoplasty is a microsurgery that requires connecting the cornea and corneal tissue of approximately 8 mm [22]. The cerebral arteries used for bypass are much smaller than the cornea. The mean diameters of the cerebral vessels are 2.35±0.60 mm (A2 segment), 1.98±0.35 mm (A3 segment), 1.76±0.36 mm (M2 segment), 1.68±0.38 mm (tonsillomedullary segment of the posterior inferior cerebellar artery), and 1.07.±0.29 mm (cortical segment of the anterior inferior cerebellar artery) [10,11]. Structural changes are more likely to occur in a cerebral artery bypass than in a keratoplasty. We hypothesized that the oblique reaction force acts 3-dimensionally at the everted suture site, generating the torque twisting the vessel.
Furthermore, given that the suture proceeded in the “right-to-left” direction downwards, the oblique reaction force likely acted counterclockwise. In the case of a “left-to-right,” downward-directed continuous suture, that is, the superior wall suture of the RCS group, the reactive torque would act in a clockwise fashion (Fig. 3). In this study, the incidence of vessel twisting was significantly different and demonstrated a significant trend: UCS followed by IS followed by RCS. The highest incidence of twisting and the greatest rotational angle in the UCS group were likely because of the synergistic counterclockwise torques generated at both suture walls. In the RCS group, absence of twisting was likely because of the torque offset by the summation of counter-directional torques generated at both suture walls. In the IS group, the torque was generated at the posterior wall only because the interrupted suture does not generate any torque (Fig. 4). We believe that the varying net torques resulted in (1) counterclockwise vessel twists in all cases and (2) significant differences in the incidence and rotation angles in our study.
The 56.7% incidence of vessel twists in the IS group suggests that even if torque is generated, the anastomosis may maintain a parallel structure depending on the thread’s tension, which is likely the reason for the absence of twisting in a sample in the UCS group. These findings suggest that the suture tension in our study was not consistent, despite a single surgeon performing the procedures. Despite this flaw, the lack of vessel twisting in the RCS group is a methodologically significant result. According to this study, the RCS technique prevented structural changes. Assuming the plausibility of our reactive torque hypothesis, vessel twist should rarely occur with the upward-directed, “right-to-left,” continuous anterior wall suture during STS bypass [20], owing to the aforementioned reasons. Also, this technique could be a way to avoid subtle technical difficulty encountered in “left-to-right” continuous suture as a right-handed surgeon.
The continuous suture is indispensable for the posterior wall suture in STS anastomosis, even for surgeons experienced in microsurgical continuous techniques. Neurovascular surgeons may struggle tremendously with deep and limited operative fields of in-situ STS bypass. Consequently, vascular neurosurgeons should practice and receive training in microsurgical continuous suture techniques and apply adequate thread tension to prevent vessel twisting.
This experimental study on the morphological differences by the suture technique is the first to compare suture techniques in microsurgical STS bypass.
Our study had several limitations. First, this is an animal and cadaveric study. In the actual surgical setting, the arteries are affixed to connective tissue unlike the freely movable samples in this study. Second, as this study was morphologic, we could not confirm the patency and complications that result from vessel twisting. Third, one junior surgeon with limited experience in bypass surgery performed all procedures, which may have introduced performance bias.
ACKNOWLEDGEMENTS
This study was approved by a local institutional review board (NON2021-002).
We thank Youngmi Kim for drawing works.
Fig. 1.
Photographs of vessel samples in this study. All twisted samples are rotated counterclockwise. (A) A 360˚-rotated sample with unidirectional continuous suture. (B) A 180˚-rotated sample with interrupted suture. (C) A non-twisted sample with the reverse continuous suture technique.
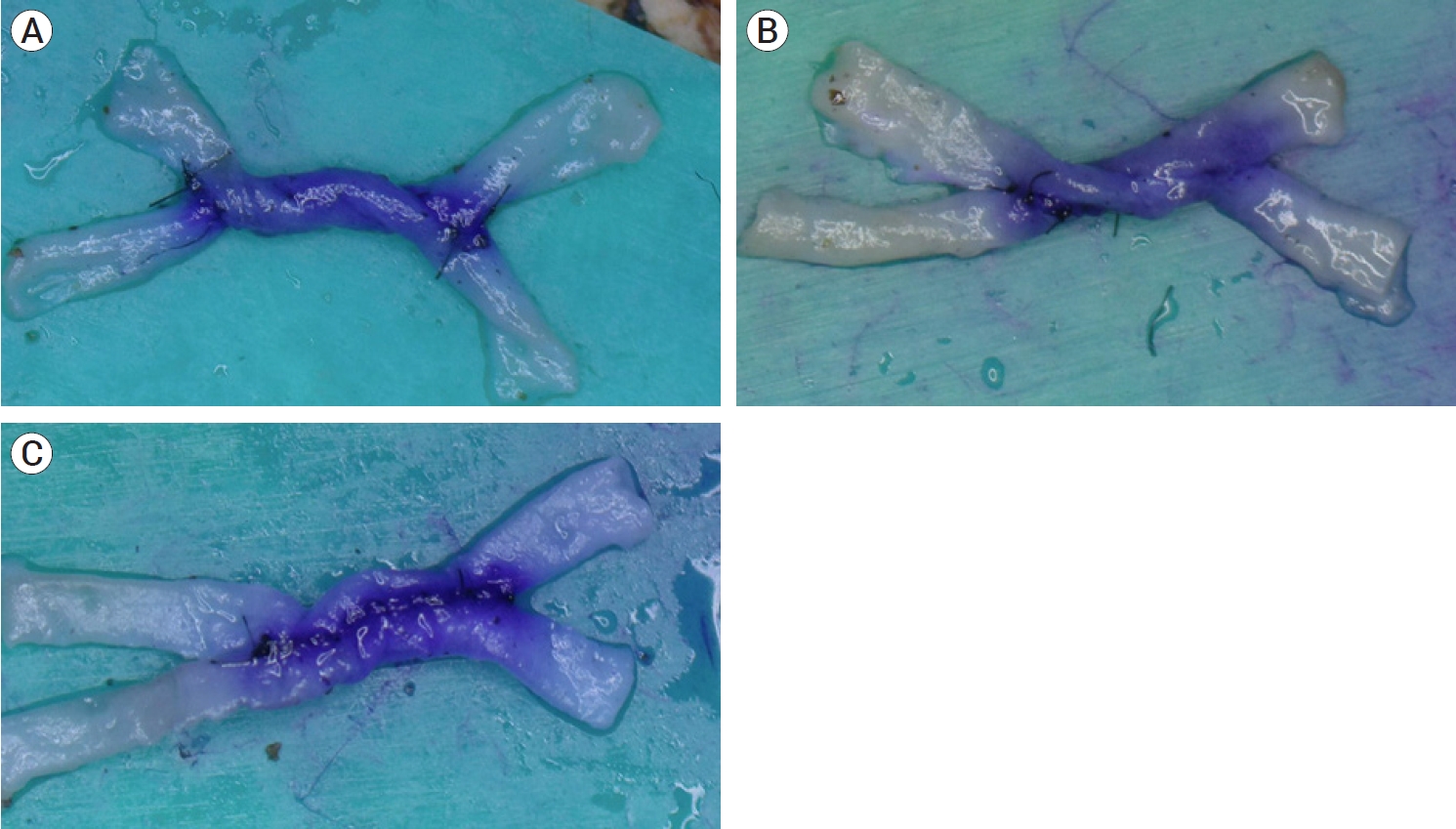
Fig. 2.
Schematic illustration of the lower stay suture knot positions in the perpendicular plane. The rotation angle was measured based on imaginary dotted line and marked as green arc angle. All samples did not twist over 360 degree in this study. (A) 0 or 360 degree, (B) 90 degree, (C) 180 degree, (D) 270 degree twisted vessels.
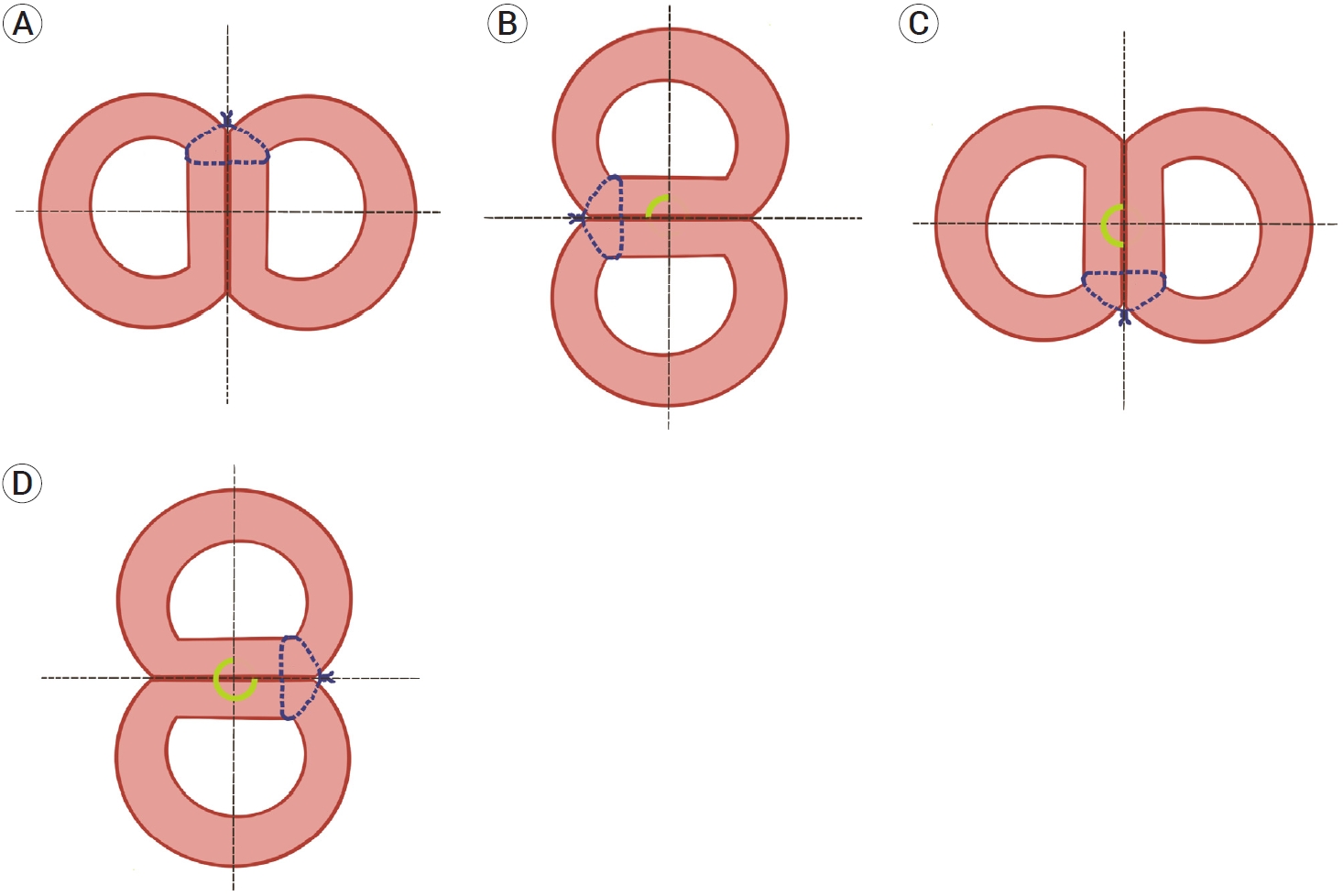
Fig. 3.
Schematic illustrations of torque generation with a “right-to-left” downward-directed continuous suture at the suture line. Thread tightening force (green arrows) and reactive force/torque (blue arrows). (A) Planar view of the suture line. (B) Three-dimensional view of the suture line. Counterclockwise reactive torques are generated at the suture line.
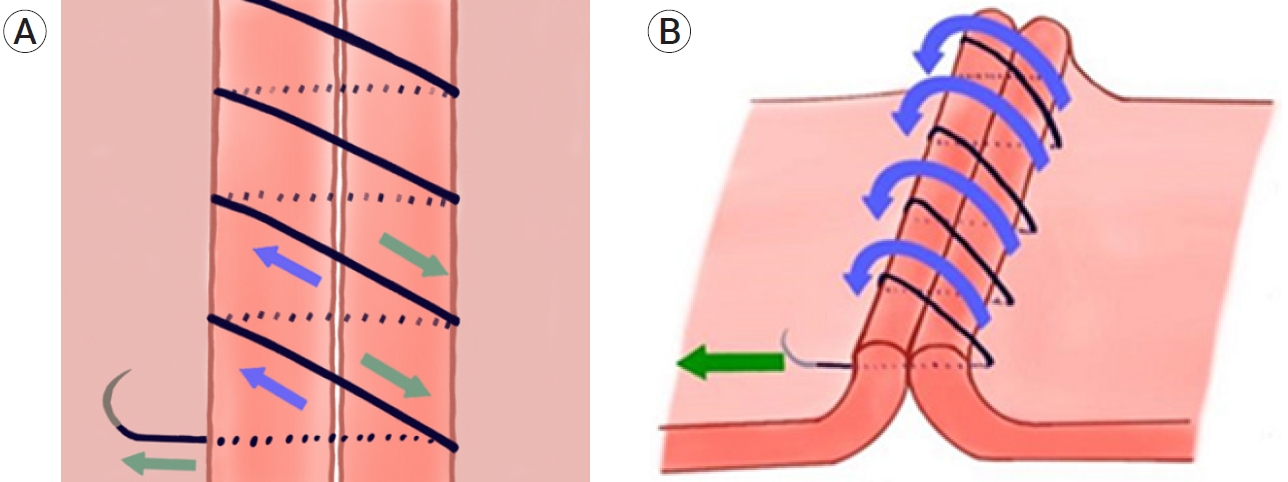
Fig. 4.
Schematic illustrations of the vector sum of the torques in the transverse view. Reactive torque (blue arrows). (A) Counterclockwise torques at both suture lines in the unidirectional continuous suture group. These torques act synergistically. (B) Opposite directional torques offset each other in the reverse continuous suture group.
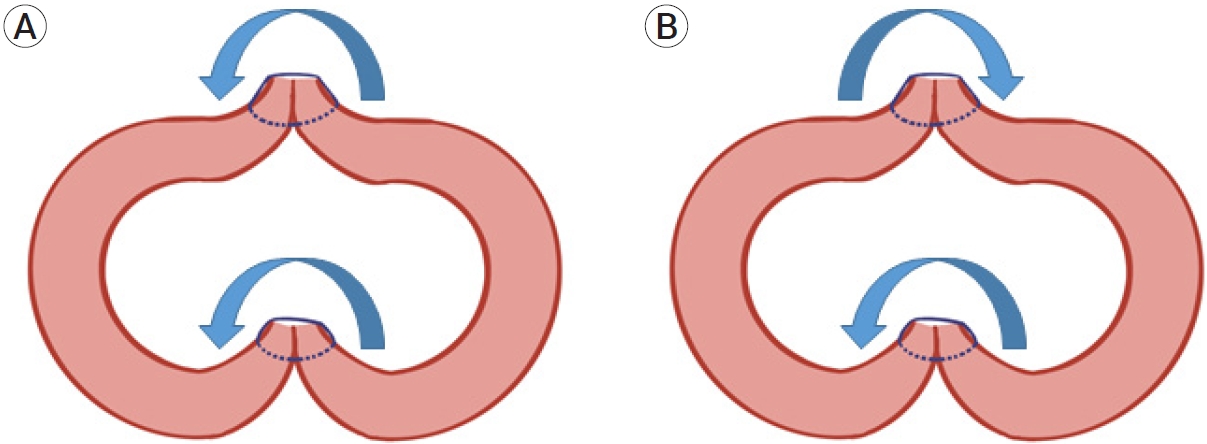
Table 1.
Outcomes in 3 different suture groups
| UCS group | IS group | RCS group | p-value | ||
|---|---|---|---|---|---|
| Vessel twisting | 29/30 (96.7) | 17/30 (56.7) | 0/30 (0) | <0.001* | |
| 0.002† | |||||
| Rotation angle (˚) | |||||
| All samples | 201±90.6 (n=30) | 102±107.6 (n=30) | 0 (n=30) | <0.001‡ | |
| <0.001§,|| | |||||
| Twisted samples | 207.9±83.7 (n=29) | 180±77.9 (n=17) | <0.001§ | ||
REFERENCES
1. Abla AA, Lawton MT. Anterior cerebral artery bypass for complex aneurysms: An experience with intracranial-intracranial reconstruction and review of bypass options. J Neurosurg. 2014 Jun;120(6):1364-77.


2. Au YK, Mahjoub SB, Hart JC. Suture patterns and corneal graft rotation in the cadaver eye. Ophthalmic Surg. 1990 Jul;21(7):472-4.


3. Bot GM, Zhao X, McElenney BK, Tayebi Meybodi A, Belykh E, Lawton MT, et al. Comparative analysis of continuous suturing, interrupted suturing, and cyanoacrylate-based lid techniques for end-to-end microvascular anastomosis: Laboratory investigation. World Neurosurg. 2020 Feb;134:465-71.


4. Chen YX, Chen LE, Seaber AV, Urbaniak JR. Comparison of continuous and interrupted suture techniques in microvascular anastomosis. J Hand Surg Am. 2001 May;26(3):530-9.


5. Grigore FN, Amin-Hanjani S. A3-A3 bypass surgery for aneurysm: Technical nuances. Oper Neurosurg (Hagerstown). 2019 Sep;17(3):277-85.


6. Hafez A, Huhtakangas J, Muhammad S, Lawton MT, Tanikawa R, Niemela M. The identification of factors that influence the quality of bypass anastomosis and an evaluation of the usefulness of an experimental practical scale in this regard. World Neurosurg. 2019 Jan;121:e119-28.


7. Hino A. Training in microvascular surgery using a chicken wing artery. Neurosurgery. 2003 Jun;52(6):1495-7; discussion 1497.



8. Kalani MY, Zabramski JM, Hu YC, Spetzler RF. Extracranial-intracranial bypass and vessel occlusion for the treatment of unclippable giant middle cerebral artery aneurysms. Neurosurgery. 2013 Mar;72(3):428-35; discussion 435.



9. Kalani MY, Zabramski JM, Nakaji P, Spetzler RF. Bypass and flow reduction for complex basilar and vertebrobasilar junction aneurysms. Neurosurgery. 2013 May;72(5):763-75; discussion 775.



10. Kawashima M, Rhoton AL Jr, Tanriover N, Ulm AJ, Yasuda A, Fujii K. Microsurgical anatomy of cerebral revascularization. Part I: Anterior circulation. J Neurosurg. 2005 Jan;102(1):116-31.


11. Kawashima M, Rhoton AL Jr, Tanriover N, Ulm AJ, Yasuda A, Fujii K. Microsurgical anatomy of cerebral revascularization. Part II: Posterior circulation. J Neurosurg. 2005 Jan;102(1):132-47.


12. Klein SR, Goldberg L, Miranda RM, Bosco P, Nelson RJ, White RA. Effect of suture technique on arterial anastomotic compliance. Arch Surg. 1982 Jan;117(1):45-7.


13. Lawton MT, Abla AA, Rutledge WC, Benet A, Zador Z, Rayz VL, et al. Bypass surgery for the treatment of dolichoectatic basilar trunk aneurysms: A work in progress. Neurosurgery. 2016 Jul;79(1):83-99.

14. Levi MA, Harb AA, Nicolas CF, Corvi JJ, Kozato A, Akelina Y, et al. Torsion is tolerated in arterial end to venous side anastomoses in the rat model. J Reconstr Microsurg. 2020 Sep;36(7):501-6.


15. Olabe J, Olabe J. Microsurgical training on an in vitro chicken wing infusion model. Surg Neurol. 2009 Dec;72(6):695-9.


16. Quinones-Hinojosa A, Lawton MT. In situ bypass in the management of complex intracranial aneurysms: Technique application in 13 patients. Neurosurgery. 2008 Jun;62(6 Suppl 3):1442-9.

17. Ramanathan D, Hegazy A, Mukherjee SK, Sekhar LN. Intracranial in situ side-to-side microvascular anastomosis: Principles, operative technique, and applications. World Neurosurg. 2010 Apr;73(4):317-25.


18. Rennert RC, Strickland BA, Radwanski RE, Ravina K, Chien M, Russin JJ. Running-to-interrupted microsuture technique for vascular bypass. Oper Neurosurg (Hagerstown). 2018 Oct;15(4):412-7.



19. Ryu J, Chung Y, Lee SH, Cho WS, Choi SK. In situ side-toside anastomosis: Surgical technique and complication avoidance. World Neurosurg. 2018 Feb;110:336-44.


20. Sanai N, Zador Z, Lawton MT. Bypass surgery for complex brain aneurysms: An assessment of intracranial-intracranial bypass. Neurosurgery. 2009 Oct;65(4):670-83; discussion 683.

21. Tayebi Meybodi A, Huang W, Benet A, Kola O, Lawton MT. Bypass surgery for complex middle cerebral artery aneurysms: An algorithmic approach to revascularization. J Neurosurg. 2017 Sep;127(3):463-79.


22. Terry MA, Li J, Goshe J, Davis-Boozer D. Endothelial keratoplasty: The relationship between donor tissue size and donor endothelial survival. Ophthalmology. 2011 Oct;118(10):1944-9.


23. Tiwari A, Cheng KS, Salacinski H, Hamilton G, Seifalian AM. Improving the patency of vascular bypass grafts: The role of suture materials and surgical techniques on reducing anastomotic compliance mismatch. Eur J Vasc Endovasc Surg. 2003 Apr;25(4):287-95.


24. Topalan M, Bilgin SS, Ip WY, Chow SP. Effect of torsion on microarterial anastomosis patency. Microsurgery. 2003 23(1):56-9.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,062 View
- 31 Download
- ORCID iDs
-
Donghwan Jeong

https://orcid.org/0000-0002-8258-3187 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



