 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(4); 2023 > Article |
|
Abstract
Pseudoaneurysms are rare but devastating complications of penetrating head traumas. They require rapid surgical or endovascular intervention due to their high risk of rupture; however, complex presentations may limit treatment options. Our objective is to report a case of severe vasospasm, flow diversion, and in-stent stenosis complicating the treatment of a middle cerebral artery pseudoaneurysm following a gunshot wound. A 33-year-old woman presented with multiple calvarial and bullet fragments within the right frontotemporal lobes and a large right frontotemporal intraparenchymal hemorrhage with significant cerebral edema. She underwent an emergent right hemicraniectomy for decompression, removal of bullet fragments, and evacuation of hemorrhage. Once stable enough for diagnostic cerebral angiography, she was found to have an M1 pseudoaneurysm with severe vasospasm that precluded endovascular treatment until the vasospasm resolved. The pseudoaneurysm was treated with flow diversion and in-stent stenosis was found at 4-month follow-up angiography that resolved by 8 months post-embolization. We report the successful flow diversion of an middle cerebral artery (MCA) pseudoaneurysm complicated by severe vasospasm and later in-stent stenosis. The presence of asymptomatic stenosis is believed to be reversible intimal hyperplasia and a normal aspect of endothelial healing. We suggest careful observation and dual-antiplatelet therapy as a justified approach.
Pseudoaneurysms are rare but devastating traumatic cerebrovascular injuries that result from the trilaminar disruption of the blood vessel wall and the formation of an encapsulated hematoma outside the lumen of the artery [21]. While less than 1% of traumatic brain injuries result in pseudoaneurysm formation, the recognition and treatment of these lesions are crucial given their friable nature leading to rupture rates and mortality as high as 50% [8,9,18]. While multiple surgical and endovascular techniques are used to treat intracranial pseudoaneurysms, there are few cases of middle cerebral artery (MCA) pseudoaneurysms treated with flow diversion [1,15]. We present a patient treated with flow diversion for an MCA pseudoaneurysm following a gunshot wound whose unique course involved severe vasospasm and in-stent stenosis.
A woman in her third decade of life presented with severe penetrating brain injury secondary to cranial-facial gunshot wounds. Head computed tomography (CT) showed multiple calvarial and bullet fragments within the right frontotemporal lobes and a large right frontotemporal intraparenchymal hemorrhage with cerebral edema (Fig. 1). She underwent decompressive hemicraniectomy and evacuation of intraparenchymal hemorrhage and accessible bone and metal fragments.
The projectile path was in proximity to the right Sylvian fissure but extensive scatter artifact on CT angiography precluded adequate evaluation of the cerebral vessels. Therefore, a diagnostic cerebral angiogram was performed on day 9 after injury once the patientŌĆÖs clinical status had stabilized. Angiography demonstrated a 3 mm pseudoaneurysm of the right M1 with severe vasospasm of the distal right internal carotid artery (ICA) and M1 segment (Fig. 2A, B). Multiple treatment options were considered including immediate or delayed flow diversion, parent artery occlusion with or without bypass, and direct surgical exploration. We felt that flow diversion offered the best chance of preserving the artery and excluding the pseudoaneurysm but that the degree of vasospasm required a delayed treatment. Therefore, daily transcranial dopplers were performed to monitor vasospasm. At 17 days follow-up, aneurysm enlargement to 4 mm was noted as well as reduction of cerebral vasospasm (Fig. 2C, D). At 19 days from injury the pseudoaneurysm grew to 4.3 mm with further resolution of vasospasm. The patient was loaded with aspirin and ticagrelor and underwent successful deployment of a Pipeline Flex Embolization Device with Shield Technology (2.5 mm├Ś12 mm) (Medtronic, MN, USA) with with measured proximal diameter of 2.1 mm and distal diameter of 1.8 mm. There was immediate decreased flow into the pseudoaneurysm and contrast stasis (Fig. 2E, F, G). She was maintained on dual-antiplatelet therapy (DAPT).
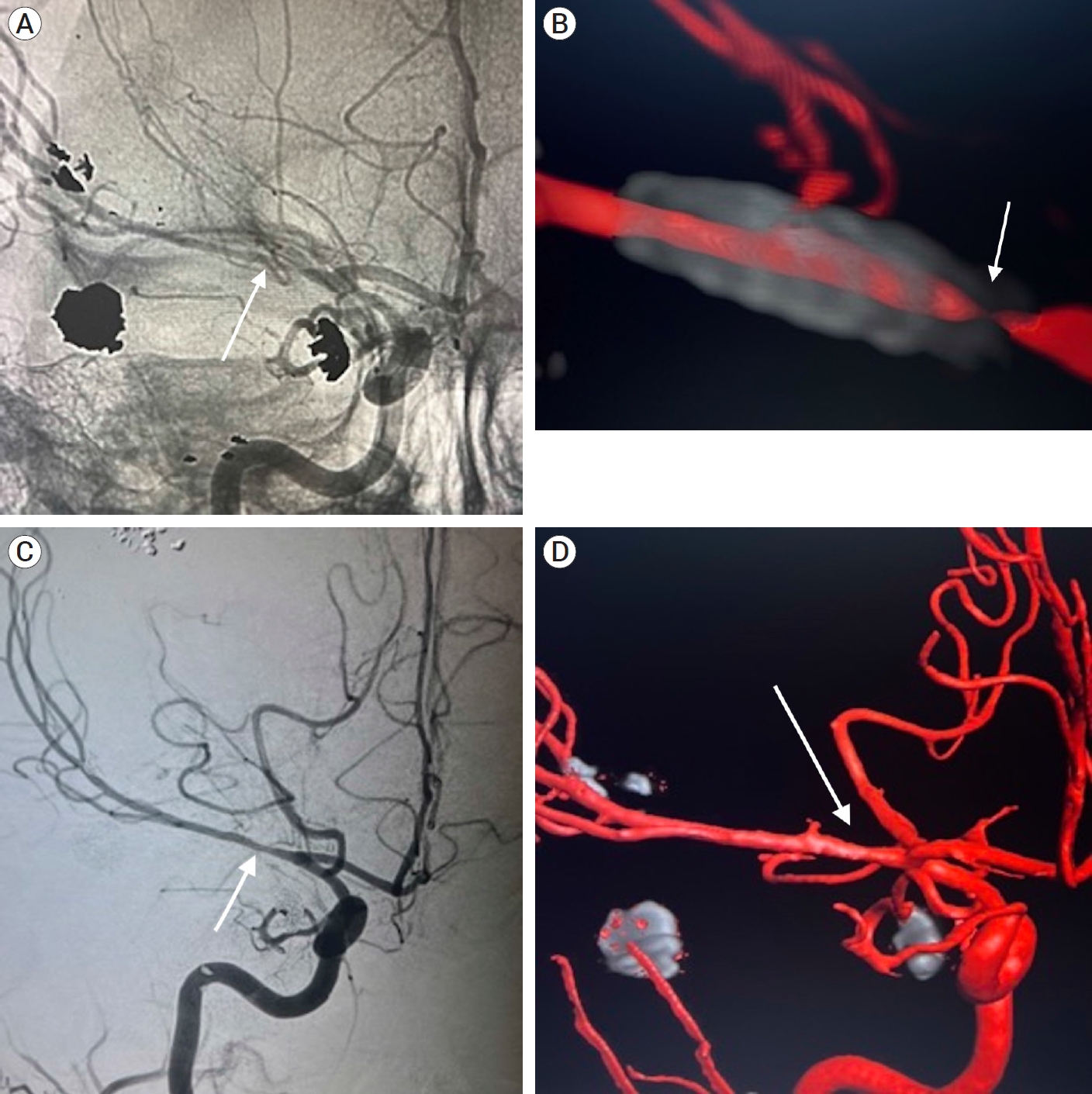
After a one-month hospital course, she was discharged to an acute rehabilitation facility. Follow-up angiography at 4 months post-embolization revealed complete occlusion of the pseudoaneurysm; however, there was significant in-stent stenosis. MCA diameter distal to the stent was 1.22 mm compared to a diameter of 0.14 mm at the proximal end of the stent (Fig. 3A, B). Although asymptomatic, she was continued on DAPT to mitigate the risk of vessel occlusion. Angiography at 8 months demonstrated marked improvement in the previously seen stenosis (Fig. 3C, D). At their most recent follow-up, the patient is able to walk with a cane with 4+/5 strength in the left lower extremity and 2/5 strength in the left upper extremity and was cleared for cranioplasty. Informed consent was verbally obtained from the patient for this case report.
Due to the high rupture risk and mortality of intracranial pseudoaneurysms, early intervention is preferrable to observation [9,21]. Microsurgical treatments include clipping, wrapping, trapping with or without bypass, and ligation of the parent artery [2,16]. While these treatments offer definitive sequestration of the pseudoaneurysm, they involve the risk of intraoperative bleeding due to pseudoaneurysm fragility, while parent vessel occlusion can lead to ischemia even with a negative balloon occlusion test [2,5,10]. Endovascular options include coiling, stent-assisted coiling, parent vessel occlusion, and flow diversion [2,3,6,14,16,17]. Flow diversion offers high rates of complete occlusion with a lower risk of pseudoaneurysm growth or intra-operative perforation as seen with coiling [1,6,13-15,17,18,20].
Several possibilities of treatment were considered with the goal of choosing the lowest risk maneuver with the greatest likelihood of aneurysmal occlusion. During the initial emergent hemicraniectomy procedure, the superior temporal artery was sacrificed and thus an extracranial to intracranial bypass utilizing the superficial temporary artery was not possible. An external carotid artery to MCA bypass using a saphenous vein graft was also considered. However, there was a serious concern that introducing a large-volume bypass graft in the presence of significant vasospasm carried unacceptable risk for hyperperfusion syndrome. Rapid increases in cerebral perfusion into a hypoperfused areaŌĆöas seen in large-volume bypass surgeries to trap intracranial aneurysmsŌĆöcan result in edema, elevated intracranial pressure, and hemorrhage [12]. Although a rare occurrence, the patientŌĆÖs hypoperfusion secondary to vasospasm led to an increased risk for hyperperfusion syndrome and therefore a large-volume bypass was deemed unsafe. As a result, we opted for an endovascular approach and favored flow diversion over coiling to minimize the risk of intraoperative perforation. The patient was carefully monitored with transcranial doppler measurements and angiography to determine the point when the MCA sufficiently relaxed to permit the safe placement of a flow-diverting device. Additionally, all preparations were made for open surgery and direct arterial reconstruction should the need arise.
The case presented here differs from the literature in a variety of ways. While the utility of flow diversion in treating ICA pseudoaneurysms has been documented in multiple case reports, the use of flow diversion in the treatment of MCA pseudoaneurysms is fairly novel [6,7,10,13,17]. Aljuboori and colleagues reported a case of an M2 pseudoaneurysm following a gunshot wound that recurred 2 weeks after coiling and was subsequently treated with flow diversion [1]. Furthermore, Scullen and colleagues treated a ruptured MCA pseudoaneurysm of iatrogenic origin with flow diversion following a complicated right lateral orbitotomy with similar success [15]. However, this is the first case report that describes the use of flow diversion as a first-line intervention for the treatment of an unruptured M1 pseudoaneurysm following a gunshot wound. Our case combined with the current literature demonstrate that flow diversion appears to be a viable option for the treatment of ruptured or unruptured MCA pseudoaneurysms.
There are additional considerations when using flow diversion in the treatment of pseudoaneurysms. The timing of intervention in our case was determined by the presence of severe post-traumatic vasospasm [19]. While delaying intervention allowed the vasospasm to subside, the pseudoaneurysm was at ongoing risk of rupture during the waiting period and indeed exhibited growth in that interval. An alternative would have been to use intra-arterial vasodilators such as verapamil to treat the vasospasm and allow earlier stent placement, which we chose not to do because of the transient effect of vasodilators. As most intracranial pseudoaneurysms are due to penetrating trauma, patients may require additional surgeries while on DAPT [9]. Lastly, a potential sequela of flow diversion is in-stent stenosis due to intimal hyperplasia [4,11]. In a study of true MCA aneurysms by Cooper et al., 60% of patients had intimal hyperplasia at 6-month follow-up, all of which were clinically asymptomatic and had improved or nearly resolved on repeat angiography within 12-18 months [4]. Our patientŌĆÖs in-stent stenosis improved markedly from 4 months to 8 months. In the presence of asymptomatic in-stent stenosis due to intimal hyperplasia, continued use of DAPT demonstrates improvement in the majority of late follow-up cases at 8-12 months and this may represent the endothelial healing response. We recommend that patients are maintained on DAPT and follow-up angiography is deferred until 12-months post-flow diversion to allow for adequate time for the resolution of intimal hyperplasia.
We report the case of MCA pseudoaneurysm formation after a gunshot wound successfully treated with flow diversion and discuss the complicating factors of post-traumatic vasospasm and transient in-stent stenosis. Flow diversion should be considered a useful option in treating MCA pseudoaneurysms.
Fig.┬Ā1.
Head CT demonstrating calvarial and bullet fragments in the right frontal lobe with large right frontal intraparenchymal hemorrhage associated with significant cerebral edema and midline shift. CT, computed tomography
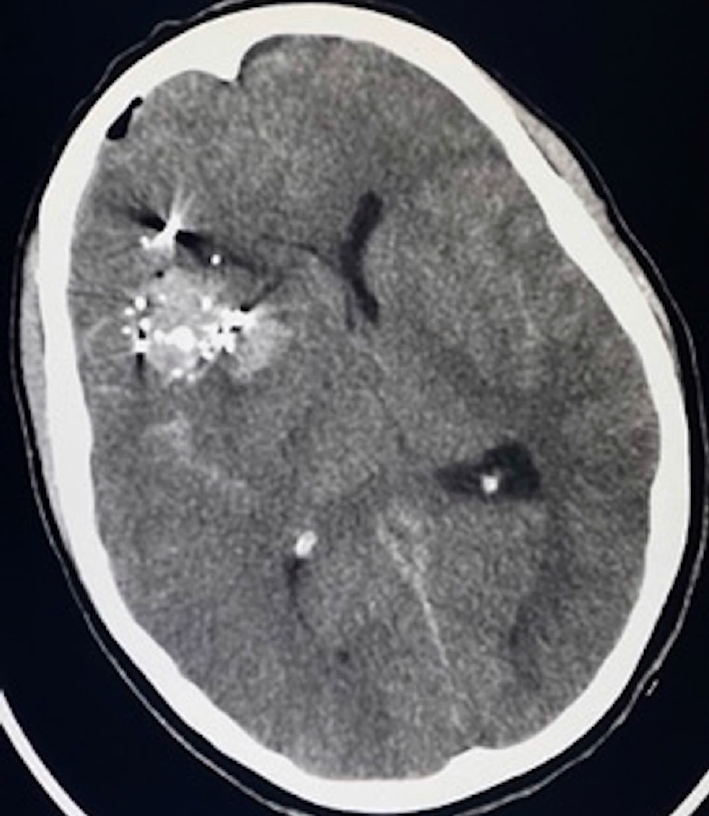
Fig.┬Ā2.
Initial AP (A) and 3D (B) cerebral angiogram demonstrating severe vasospasm within the distal right ICA and right M1 segment with a 3 mm right M1 pseudoaneurysm. Follow-up AP (C) and 3D (D) cerebral angiogram demonstrating improved distal right ICA and MCA vasospasm with the right M1 pseudoaneurysm measuring 4 mm in diameter. (E) AP cerebral angiogram pre-pipeline embolization demonstrating continued improvement in distal right ICA and MCA vasospasm with the right M1 pseudoaneurysm now measuring 4.3 mm in diameter. (F) AP cerebral angiogram post-pipeline embolization of right M1 pseudoaneurysm with immediate stasis and decreased filling. (G) Vaso CT post-pipeline embolization. ICA, internal carotid artery; MCA, middle cerebral artery; CT, computed tomography
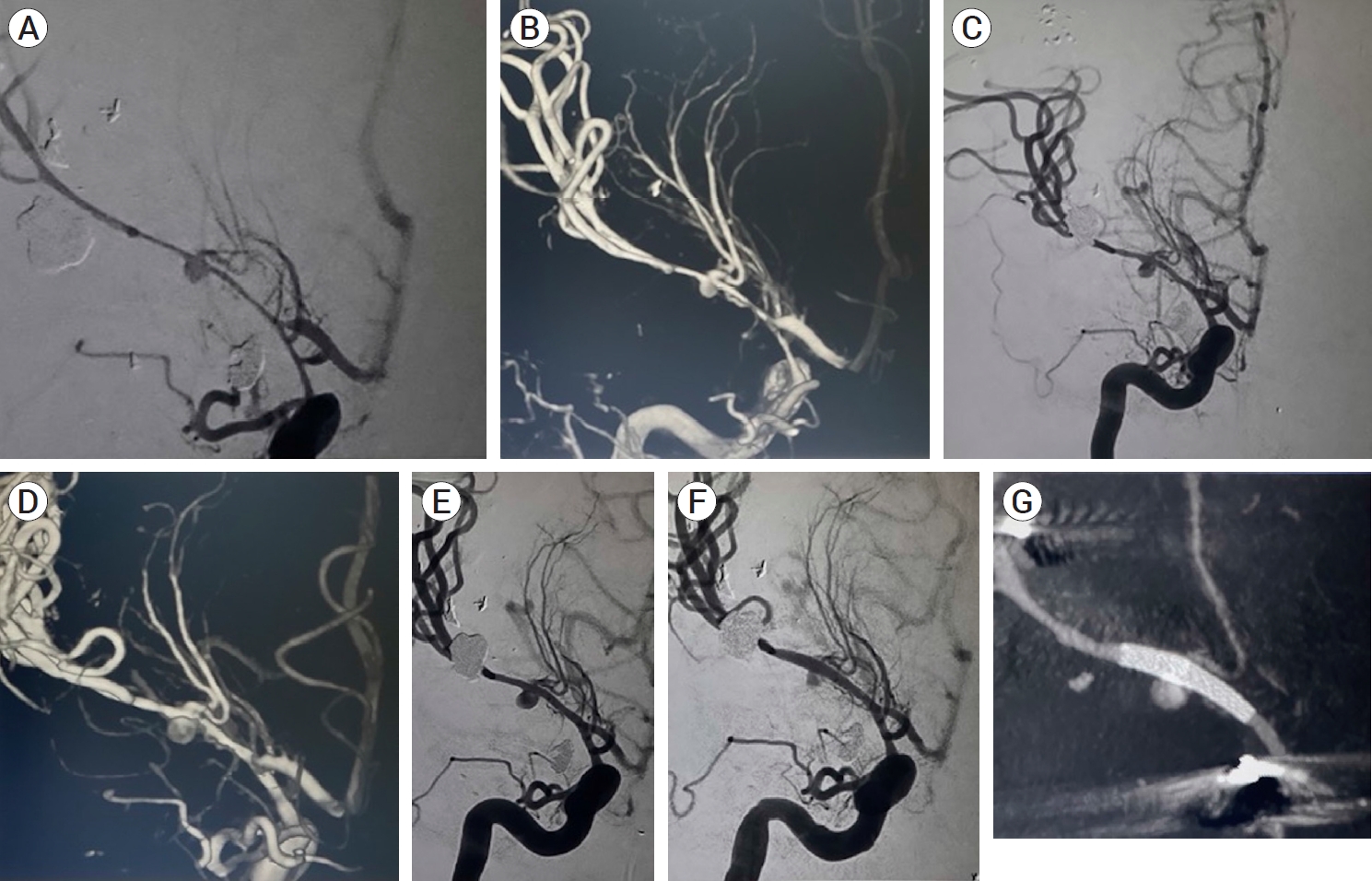
Fig.┬Ā3.
Unsubtracted AP (A) and 3D (B) dual volume cerebral angiogram demonstrating no further filling of the right M1 pseudoaneurysm with severe in-stent stenosis of the proximal pipeline. 8-month follow-up AP (C) and 3D (D) cerebral angiogram demonstrating significantly improved in-stent stenosis with no further filling of the right M1 pseudoaneurysm. White arrows point to the location of the flow-diverting stent in the proximal M1.
REFERENCES
1. Aljuboori Z, Meyer K, Ding D, James R. Endovascular treatment of a traumatic middle cerebral artery pseudoaneurysm with the pipeline flex embolization device. World Neurosurg. 2020 Jan;133:201-4.


2. Anderson E, Chalouhi N, Dumont A, Tjoumakaris S, Zanaty M, Rosenwasser R, et al. Management of head and neck pseudoaneurysms: A review of 33 consecutive cases. The Scientific World Journal. 2014 2014:419803.




3. Cohen JE, Gomori JM, Segal R, Spivak A, Margolin E, Sviri G, et al. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. 2008 Sep;63(3):476-85; discussion 485. -6.



4. Cooper JB, Greisman JD, Dakay K, Kaur G, Al-Mufti F, Gandhi CD, et al. Incidence of neo-intimal hyperplasia in anterior circulation aneurysms following pipeline flow diversion. J Stroke Cerebrovasc Dis. 2021 Jul;30(7):105794.


5. Elias AE, Chaudhary N, Pandey AS, Gemmete JJ. Intracranial endovascular balloon test occlusion: Indications, methods, and predictive value. Neuroimaging Clin N Am. 2013 Nov;23(4):695-702.

6. Ghorbani M, Shojaei H, Bavand K, Azar M. Surpass streamline flow-diverter embolization device for treatment of iatrogenic and traumatic internal carotid artery injuries. AJNR Am J Neuroradiol. 2018 Jun;39(6):1107-11.



7. Ghorbani M, Wipplinger C, Griessenauer CJ, Shojaei H, Abdolhoseinpour H. Successful endoluminal reconstruction of a pseudoaneurysm of the internal carotid artery following a transorbital stab injury. Clin Neurol Neurosurg. 2020 Jul;194:105838.


8. Horiuchi T, Nakagawa F, Miyatake M, Iwashita T, Tanaka Y, Hongo K. Traumatic middle cerebral artery aneurysm: Case report and review of the literature. Neurosurg Rev. 2007 Jul;30(3):263-7; discussion 267.



9. Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000 Jan;8(1):1-6.


10. Lee H, Perry JJ, English SW, Alkherayf F, Joseph J, Nobile S, et al. Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2018 Jun;1-8.

11. Luecking H, Doerfler A, Goelitz P, Hoelter P, Engelhorn T, Lang S. Two- to five-year follow-up of 78 patients after treatment with the flow redirection endoluminal device. Interv Neuroradiol. 2020 Feb;26(1):38-44.




12. Mohri M, Ichinose T, Uchiyama N, Misaki K, Nambu I, Takabatake Y, et al. Hyperperfusion syndrome after trapping with high-flow bypass for a giant paraclinoid internal carotid artery aneurysm. World Neurosurg. 2018 Jul;115:143-6.


13. Rajah G, Garling RJ, Rangel-Castilla L. Large traumatic skull base internal carotid artery pseudoaneurysm managed with endovascular flow diversion: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2018 Dec;15(6):e84.



14. Sastry RA, Koch MJ, Grannan BL, Stapleton CJ, Butler WE, Patel AB. Flow diversion of a recurrent, iatrogenic basilar tip aneurysm in a pediatric patient: Case report. J Neurosurg Pediatr. 2018 Jan;21(1):90-3.


15. Scullen T, Mathkour M, Carr JR, Dumont AS, Amenta PS. Iatrogenic middle cerebral artery ruptured pseudoaneurysm successfully treated with a pipeline embolization device. Ochsner J. 2021 Summer;21(2):190-3.



16. Shi Y, Gao Y, Liu Y, Cui W, Zhou G, Wang L, et al. Treatment of traumatic intracranial pseudoaneurysms: A single-center experience. Front Neurol. 2021 Jun;12:690284.



17. Tan S, Zhou X, Lu Y, Lai L, Huang X, Li B, et al. Low-profile visualized intraluminal support stent for the endovascular treatment of traumatic intracranial internal carotid artery pseudoaneurysms. Neurosurg Rev. 2022 Jun;45(3):2231-7.



18. Tunthanathip T, Phuenpathom N, Sae-Heng S, Oearsakul T, Sakarunchai I, Kaewborisutsakul A. Traumatic cerebrovascular injury: Clinical characteristics and illustrative cases. Neurosurg Focus. 2019 Nov;47(5):e4.

19. Weidauer S, Lanfermann H, Raabe A, Zanella F, Seifert V, Beck J. Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2007 Jun;38(6):1831-6.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,524 View
- 32 Download
- ORCID iDs
-
Christopher S. Ogilvy

https://orcid.org/0000-0003-4600-8545 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



