 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(3); 2023 > Article |
|
Abstract
Objective
The term “weekend effect” refers to an increase in the mortality rate for hospitalizations occurring on weekends versus weekdays. In this study, we investigated whether such an effect exists in patients undergoing mechanical thrombectomy for acute ischemic stroke with large vessel occlusion (currently the standard treatment for this condition) at a single center in Japan.
Methods
We surveyed 151 patients who underwent mechanical thrombectomy for acute ischemic stroke with large vessel occlusion (75 and 76 patients were treated during daytime and nighttime, respectively) from January 2019 to June 2021. The items evaluated in this analysis were the rate of modified Rankin Scale ≤2 or prestroke scale, mortality, and procedural treatment time.
Results
The rates of modified Rankin Scale ≤2 or prestroke scale and mortality at 90 days after treatment did not differ significantly between daytime and nighttime (41.3% vs. 29.0%, p=0.11; 14.7% vs. 11.8%, p=0.61, respectively). The door-to-groin time tended to be shorter during daytime versus nighttime (57 [IQR: 42.5-70] min vs. 70 [IQR: 55-82]) min, p=0.0507).
Numerous studies have demonstrated that mechanical thrombectomy (MT) is a very effective treatment and the current standard therapy for acute ischemic stroke (AIS) caused by large vessel occlusion (LVO) [5,6,8,9,11,19]. The term “weekend effect” refers to an increase in the mortality rate for hospitalizations occurring on weekends versus weekdays [3]. This effect has also been reported in the field of AIS [2].
Recently, studies investigating the potential existence of the weekend effect with regard to MT for AIS caused by LVO have been reported [4,16,23]. These studies have yielded varied results, such as no difference in outcome between weekdays and weekends [16], higher mortality rates at nighttime and on weekends [23], and better outcome on weekends [4]. Considering these findings, the existence of the weekend effect for MT appears to be controversial.
In this study, we examined the potential existence of differences in MT for AIS induced by LVO performed between daytime and nighttime in a Japanese institution and compared the results with those reported in other countries.
This is a retrospective, single-center study. We evaluated patients who underwent MT for LVO from January 2019 to June 2021. Eligible patients with AIS were selected based on the current guidelines for MT established by the American Heart Association/American Stroke Association [17,18]. This study was approved by the institutional review board of our institution, and preoperative consent was obtained from all patients who participated in the study.
According to the DAWN and DEFFUSE-3 trials [1,15], the treatment indication time is 6-24 hours after the onset. Notably, LVO was diagnosed through computed tomography angiography (CTA) or magnetic resonance imaging (MRI) and angiography and was treated without perfusion imaging. However, some patients transferred from other hospitals were treated with expanded indications. Additionally, some patients with Alberta Stroke Program Early CT score (ASPECTS) or Diffusion Weighted Imaging-ASPECTS (DWI-ASPECTS) <6 were treated with expanded indications following a request from the families of patients. Among those treated with expanded indications, there were several patients with prestroke modified Rankin Scale (mRS) ≥3.
Cases of MT for LVO that occurred in hospital and during other endovascular treatments were excluded. The patients included in this study were divided into a daytime group and a nighttime group. The daytime group referred to MT performed from 7:00 to 19:00 on weekdays (Monday to Friday), while the nighttime group referred to MT performed on weekdays from 19:00 to 7:00 the next day, weekends, and holidays.
Our institution is certified as a primary stroke core center by the Japan Stroke Association and supports local stroke care. Our neurointervention team during nighttime consists of two neurosurgeons on duty every day. Following the decision to perform MT, the on-call or duty neurointervention physician is called. The preparation and treatment of LVO is often performed by the neurointervention physician alone. The night shift nurse team consists of two nurses until 21:00. After 21:00, an on-call nurse is called when performing MT. During this time, MT is prepared by the nurse together with the nurse managing the night shift (Fig. 1). In our hospital, the emergency, computed tomography, magnetic resonance imaging, and angiography rooms are adjacent or facing each other. This minimizes the time required to move patients between rooms. All procedures were performed via the transfemoral approach under local anesthesia. MT for anterior circulation was performed with a 9-French balloon guiding catheter positioned within the ipsilateral internal carotid artery (ICA), and a distal access aspiration catheter and a microcatheter were used as appropriate. Collected data included patient demographics (i.e., age, sex), risk factors and etiology detected after MT, neurologic examination using the National Institutes of Health Stroke Scale, use of intravenous recombinant tissue-type plasminogen activator (iv rt-PA), obstructed location, and imaging score (DWI-ASPECTS or ASPECTS) on presentation. AIS etiology was speculated to be embolus if there was no stenosis in the lesion reopened by MT, ischemia was observed in the cortex, and atrial fibrillation was detected on electrocardiogram. Treatment metrics were also recorded, including door-to-groin, groin-to-recanalization, and door-to-recanalization times, as well as MT success based on the Thrombolysis In Cerebral Infarction score at the final angiography. Outcome data, including 90-day functional outcome (based on the mRS or prestroke scale) and mortality at 7 and 90 days, were also collected. In addition, in a subgroup analysis, the items described above were evaluated in patients with occluded ICA or M1. Continuous variables were reported as the median (interquartile range [IQR]). Univariate statistical analysis was performed using the Wilcoxon signed-rank test for continuous variables and the Pearson’s chi-square test for categoric variables. All statistical analyses were performed using the Microsoft ExcelⓇ 2016 software (USA). In all analyses, p<0.05 denoted statistically significant differences.
A total of 154 MT procedures were performed during the study period. There were two patients with in-hospital onset and one patient with intraoperative embolism; these patients were excluded from the analysis. Of the remaining 151 patients, 75 and 76 patients underwent MT during daytime and nighttime, respectively. Table 1 shows the baseline demographics, stroke risk factors and etiology, occluded vessels, and transfer cases of the daytime and nighttime groups. In this study, there were significantly fewer male patients in the daytime group compared with the nighttime group (44% vs. 61.8%, respectively, p=0.028). No difference in the rate of using iv rt-PA was observed between the two groups (25.3% vs. 30.2%, respectively, p=0.49).
The proportion of patients with diabetes mellitus or dyslipidemia was higher in the daytime group compared with the nighttime group (27% vs. 7.9%, p=0.002; 28% vs. 13.2%, p=0.024, respectively). There was no significant difference between the two time periods in DWI-ASPECTS. However, the score tended to be higher in the daytime group versus the nighttime group (8 [IQR: 6-9] vs. 7 [IQR: 6-8.25], respectively, p=0.071).
Table 2 shows treatment procedure times and postoperative functional outcomes. The door-to-groin time tended to be shorter in the daytime group than in the nighttime group, and the difference was slightly not statistically significant (57 [IQR: 42.5-70] vs. 70 [IQR: 55-82] min, respectively, p=0.0507). Other procedural times, final angiography, post treatment outcome, and mortality at 7 and 90 days did not differ between daytime and nighttime. Of the 9 deaths occurred within 7 days after MT, 4 were due to brain swelling after extensive cerebral infarction such that 1 each for brainstem infarction, hemorrhagic infarction, acute heart failure, infection, and subarachnoid hemorrhage during MT.
Table 3 indicates the profile and procedural time, final angiography, and postoperative outcome of treated patients with occluded ICA or M1. This subgroup analysis included 110 patients (54 and 56 patients from the daytime group and nighttime group, respectively). The rate of patients with diabetes mellitus was higher in the daytime group than the nighttime group (28.6% vs. 3.7%, respectively, p=0.0004). Other patient profiles did not differ between the two groups. In this subgroup analysis, the door-to-groin time was significantly shorter in the former group than the latter group (54 [IQR: 40.75-64] vs. 65.5 [IQR: 54-80] min, respectively, p=0.037). Nevertheless, groin-to-recanalization and door-to-recanalization times did not differ between the two time periods (groin-to-recanalization: 54 [IQR: 29.5-79.5] vs. 68 [IQR: 34.5-88.5] min, p=0.12; door-to-recanalization: 113 [IQR: 79.5-144.5] vs. 129 [IQR: 94.5-170.5] min, p=0.12, respectively). Moreover, there were no differences between the two groups in final angiography findings, postoperative outcome, and mortality.
MT has shown efficacy for the treatment of AIS with LVO and has been established as a standard therapeutic option for this condition [5,6,8,9,11,19]. This treatment has also become indispensable in Japan based on the highlevel evidence included in the guideline established by the Japan Stroke Society [21]. For more than 20 years, the term “weekend effect” has been used by the medical community to describe the higher mortality rate for hospitalizations occurring on weekends versus weekdays [3]. This effect has been recognized in the fields of cancer, pulmonary embolism, and stroke [2,13]. Several studies examined the weekend effect in ischemic stroke requiring endovascular MT. These reported yielded conflicting results; thus, the presence of the weekend effect is controversial [4,16,23]. Treatment outcomes may vary depending on the country, facility level, and patient population. In the present study conducted in Japan, we investigated whether performing MT for AIS with LVO during daytime and nighttime influences the treatment time and outcome. Our data did not show differences in MT outcome and mortality between daytime and nighttime. In addition, a subanalysis of ICA and M1 cases alone showed that the door-to-groin time was shorter during daytime than nighttime; however, there was no difference in treatment outcome. This evidence demonstrated that the approach to the treatment of AIS in our institution was effective, even with shortage of manpower. The median age of patients in this study was 79 years. This is slightly higher than the average or median age of 71-75 years reported in previous studies examining the weekend effect, although statistical analysis was not performed (Table 4). This result indicates that the patients treated with MT for AIS are older in this study than the rest of the world due to the country’s aging society. Furthermore, it has been reported that the outcomes of MT are worse in older patients versus younger patients [10]. The mRS ≤2 or prestroke scale at 90 days after MT was 34.9%, despite the large number of elderly patients in this study. This result is acceptable considering that the range of rates reported in other countries is 30-44%. In addition, door-to-groin time was 20-30 minutes shorter than that reported in other countries for both daytime and nighttime. Furthermore, the door-to-recanalization time was 15-40 minutes shorter. This speed may affect the outcomes in elderly patients. Our institution has an emergency room, an imaging laboratory, and an angiography room adjacent to each other. The extremely short travel time between these rooms is probably a major advantage. The prevalence of diabetes mellitus was higher during the daytime, and other risk factors were also more prevalent, although not significantly different. Our guess is that daytime admissions may have allowed more time for the family of patients to hear past history of patients, and more experienced staff were often able to correspond to them.
The fact that there was no difference in outcomes between the two groups is great, but there are things we would like to work on to further improve the outcomes. The first is about the use of iv rt-PA. Compared with other countries, the rate of using iv rt-PA in this study was small. This result discouraged the use of iv rt-PA because of the concerns about hemorrhagic complication with iv rt-PA, and the concept of earlier recanalization with MT alone would be better. There are reports of non-inferiority of MT alone compared to MT wit iv rt-PA [22,24]. On the other hand, some reports that MT alone did not show non-inferiority compared to MT with iv rt-PA [7,12,14]. For these reasons, it seems advisable to use MT and iv rt-PA in combination as per the guideline at this time. Considering the reported the rate of emboli to distal and new territories related to MT was 40% [20], iv rt-PA may be expected to be effective against such emboli. In the future, we may need to shift to iv rt-PA before and during MT in cases that indicated iv rt-PA, especially in cases that took a long time to puncture. In addition, it may be possible to shorten the door-to-puncture time by reducing the quality of MRI images or by narrowing the MRA scan area. The images required for MT are not highly precise images, a quick and rough image is sufficient, so we would like to develop an imaging protocol and work on it. However, there are many limitations of this study. This study was a retrospective and single center analysis of patients who underwent MT for AIS. The presence of weekends effect varied depending on the medical system of countries and regions. As the primary stroke core center in Japan, it is important to note that there was no significant difference between the two groups in this study. The analysis included only patients who had undergone MT, and there was a selection bias. There were also some differences in patient profiles, the causes of which are unknown.
This single primary stroke core-center analysis showed that the outcome and mortality rate associated with MT for the treatment of AIS did not differ between daytime and nighttime, and there was no “weekend effect.” Despite the presence of more elderly patients in this study compared with other countries, we were able to achieve world-class treatment results by shortening the door-to-recanalization time.
Fig. 1.
This figure shows staff to MT during the nighttime zone (White circle: A neurosurgeon and nurse on duty, Black circle: A neurointervention physician, Gray circle: nurse until 21:00, White triangle: A nurse managing the night shift, Black triangle: An on-call nurse). LVO, large vessel occlusion; MT, mechanical thrombectomy
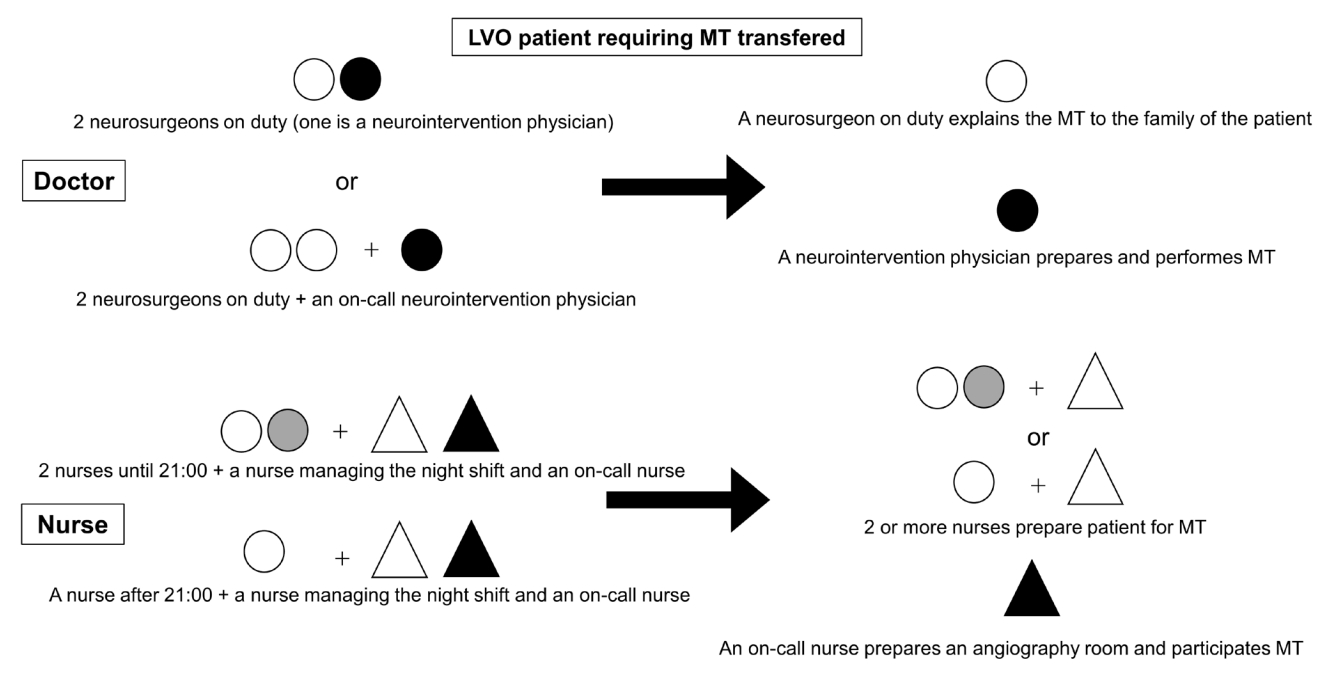
Table 1.
Univariate analysis of the characteristics of patients who underwent mechanical thrombectomy during daytime and nighttime
Categoric variables are expressed as number (%), and continuous variables, as median (IQR). NIHSS, National Institutes of Health Stroke Scale; MRI, magnetic resonance imaging; CTA, computed tomography angiography; DWI-ASPECTS, Diffusion Weighted Imaging-Alberta Stroke Program Early CT Score; mRS, modified Rankin Scale; ICA, internal carotid artery
Table 2.
Procedural times and functional outcomes
Table 3.
Univariate analysis of the characteristics, procedural treatment times, and outcomes of patients who underwent mechanical thrombectomy only on large vessels of anterior circulation during daytime and nighttime
Table 4.
Comparison of studies examining the weekend effect of MT for AIS
REFERENCES
1. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018 Feb;378(8):708-18.



2. Albright KC, Raman R, Ernstrom K, Hallevi H, Martin-Schild S, Meyer BC, et al. Can comprehensive stroke centers erase the ‘weekend effect’? Cerebrovasc Dis. 2009 27(2):107-13.



3. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001 Aug;345(9):663-8.


4. Benali A, Moynier M, Dargazanli C, Deverdun J, Cagnazzo F, Mourand I, et al. Mechanical thrombectomy in nighttime hours: Is there a difference in 90-day clinical outcome for patients with ischemic stroke? AJNR Am J Neuroradiol. 2021 Mar;42(3):530-7.



5. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015 Jan;372(1):11-20.

6. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015 Mar;372(11):1009-18.


7. Fischer U, Kaesmacher J, Strbian D, Eker O, Cognard C, Plattner PS, et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: An open-label, blinded-outcome, randomised non-inferiority trial. Lancet. 2022 Jul;400(10346):104-15.

8. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015 Mar;372(11):1019-30.


9. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016 Apr;387(10029):1723-31.


10. Hendrix P, Killer-Oberpfalzer M, Broussalis E, Melamed I, Sharma V, Mutzenbach S, et al. Outcome following mechanical thrombectomy for anterior circulation large vessel occlusion stroke in the elderly. Clin Neuroradiol. 2022 Jun;32(2):369-74.



11. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015 Jun;372(24):2296-306.


12. LeCouffe NE, Kappelhof M, Treurniet KM, Rinkel LA, Bruggeman AE, Berkhemer OA, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021 Nov;385(20):1833-44.

13. Mekonnen B, Wang G, Rajbhandari-Thapa J, Shi L, Thapa K, Zhang Z, et al. Weekend effect on in-hospital mortality for ischemic and hemorrhagic stroke in US rural and urban hospitals. J Stroke Cerebrovasc Dis. 2020 Oct;29(10):105106.



14. Mitchell PJ, Yan B, Churilov L, Dowling RJ, Bush SJ, Bivard A, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: An open-label, blinded-endpoint, randomised non-inferiority trial. Lancet. 2022 Jul;400(10346):116-25.


15. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018 Jan;378(1):11-21.

16. Potts MB, Abdalla RN, Golnari P, Sukumaran M, Palmer AH, Hurley MC, et al. Analysis of mechanical thrombectomy for acute ischemic stroke on nights and weekends versus weekdays at comprehensive stroke centers. J Stroke Cerebrovasc Dis. 2021 Apr;30(4):105632.


17. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015 Oct;46(10):3020-35.


18. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018 Mar;49(3):e46-110.


19. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015 Jun;372(24):2285-95.


20. Wong GJ, Yoo B, Liebeskind D, Baharvahdat H, Gornbein J, Jahan R, et al. Frequency, determinants, and outcomes of emboli to distal and new territories related to mechanical thrombectomy for acute ischemic stroke. Stroke. 2021 Jul;52(7):2241-9.


21. Yamagami H, Hayakawa M, Inoue M, Iihara K, Ogasawara K, Toyoda K, et al. Guidelines for mechanical thrombectomy in Japan, the fourth edition, March 2020: A guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo). 2021 Mar;61(3):163-92.



22. Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020 May;382(21):1981-93.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,001 View
- 29 Download
- ORCID iDs
-
Naoki Omura

https://orcid.org/0000-0002-5962-4825 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



