|
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(4); 2023 > Article |
|
Abstract
Objective
Optochiasmatic cavernoma is an extremely rare cerebral lesion. They account for approximately 1% of all cavernomas of the central nervous system. Reports on this pathology are limited. Abrupt visual deterioration is a common symptom of the disease. Treatment strategy and visual outcomes after different treatment approaches remain a subject for discussion.
Methods
Patients operated in a period 2005-2021 were analyzed in this study. All patients preoperatively underwent computed tomography (CT) scan, CT-angiography, and magnetic resonance imaging (MRI). Visual function of the patients was assessed pre-op, post-op and at the follow-up. Duration of visual dysfunction was noted as well. Surgical details were also extracted from medical notes. All patients were followed up, and control MRI was performed one month after operation. We assessed surgical series of optochiasmatic cavernomas published for last 10 years. Further comparative analysis with our data was performed.
Results
Five patients were included into this study. There were four men and one woman. Mean age comprised 33.8 years (range 20-48 years). Most patients were admitted to our hospital due to visual disturbances (80%). Visual function improved in four patients. Visual function was unchanged in one patient, lacking visual disturbancies pre-op. Complication developed in one patient.
Conclusions
Optochiasmatic cavernomas are encountered extremely rare. Despite the use of contemporary diagnostic options, differential diagnosis remains challenging. Full diagnostic work-up is mandatory. After the diagnosis is made, surgical treatment should be considered first. Total microsurgical or endoscopic transsphenoidal removal of the optochiasmatic cavernoma is a relatively safe and effective treatment method facilitating improvement of visual function.
Cavernomas of the central nervous system are benign low-flow vascular malformations that are encountered in 0.2-0.5% of the population with a small female predominance [5,13,20]. They comprise 10-20% of vascular malformations of the central nervous system [6,7,9]. Cavernomas consist of dilated pathological vessels (caverns) lined by endothelium, and there is no brain tissue in between [3,10,12]. Most cavernomas are found in the basal ganglia, cerebellum, brainstem, and spinal cord: 80% are supratentorial, 20% - infratentorial [14-16].
Optochiasmatic cavernoma is an extremely rare cerebral lesion. They account for approximately 1% of all cavernomas of the central nervous system [13]. Reports on this pathology are limited. 118 cases have been reported since 1958 [20]. It is a very rare cause of visual symptoms and findings caused by optic nerve dysfunction. Abrupt visual deterioration is a common symptom of the disease which is sometimes predisposed by factors like alcohol consumption, pregnancy, labour etc [5,13,20]. Visual deterioration can be either due to enlargement or abrupt bleeding of cavernoma. Hemorrhage can be either intracavernoma or extra cavernoma. However, signs of pituitary hypofunction and hydrocephalus can be encountered in certain cases due to significant mass-effect [6,7,9]. Magnetic resonance imaging is the imaging modality of choice for preoperative diagnosis of cavernoma. Preoperative diagnosis, treatment strategy and visual outcomes after different treatment modalities remain a subject for discussion. Hereby, we present clinical and radiographic features as well as surgical results of a series of five patients with optochiasmatic cavernomas.
Patients operated in a period 2005-2021 were analyzed in this study. All patients preoperatively underwent computed tomography (CT) scan, CT-angiography, and magnetic resonance imaging (MRI).
All cavernomas had heterogeneous features on T1WI, most of the lesion was isointensive with small hyperintensive sites (due to minor hemorrhage).
MRI diagnosis was complicated in cases when no features of local hemorrhage could be recognized. In one patient with large cavernoma (patient 1), lesion was mostly exophytic and extended to the third ventricle and the left lateral ventricle. Thus, the cavernoma was misdiagnosed as a craniopharyngioma in this case.
The cavernoma was displaced anteriorly in another patient (patient 5) and had low-intensity signal on FIESTA. For these reasons, meningioma of the anterior clinoid process was proposed initially.
Visual function of the patients was assessed pre-op, post-op and at the follow-up. Duration of visual dysfunction was noted as well. Surgical details were also extracted from medical notes. All patients were followed up, and control MRI was performed one month after operation.
We assessed surgical series of optochiasmatic cavernomas published for last 10 years. Further compare of results with our data was performed.
In this study patients underwent microsurgical, and endoscopic transnasal removal of the cavernomas (Table 1). Surgical strategy was based on the location of the cavernoma and diagnostic work up. In the case when craniopharyngioma was suspected, the lesion was located in the posterior superior portion of the chiasm with extension to the third ventricle. For these reasons transnasal endoscopic approach was selected for this patient. Remaining lesions were mostly located in the anterior part of the chiasm and the optic nerve. Location of cavernomas is depicted schematically in Fig. 1.
Microsurgical removal was performed in four patients. Standard pterional craniotomy and subfrontal approach were used in all these four cases. Side of the approach was determined by the location of a cavernoma and a concomitant intracranial lesion. After subarachnoid dissection optic structures were identified. Cavernoma looked like a xanthochromic area on the surface of the optic apparatus and caused its local bulging. Internal structure of cavernomas was atypical in all cases. It consisted of few tiny venous vessels not resembling standard caverns that are encountered in typical cavernomas. Cavernomas were surrounded by some extent of a dense perifocal gliosis. However, no clear boundaries were found between the lesion and surrounding normal brain tissue. Cavernomas were removed according to standard microsurgical principles. Cavernoma-related hematoma was encountered in 3 cases. However, it lacked typical capsule. The hematoma was in the subpial space in the ventral chiasm in first patient. It was found in the upper part of the chiasm next to the lamina terminalis in another case. The hematoma in third ventricle was found in third case.
As mentioned above, endoscopic transnasal removal of a cavernoma was performed in one patient. The anterior extended transsphenoidal route was used. Intraoperative visual evoked potentials were also applied. The dura was opened with linear incision at the sella and the planum sphenoidale. Almost intact chiasm was found at the planum. Given preoperative sodium balance disturbances, pituitary transection was considered unharmful. The pituitary stalk was transected as well to increase the operative corridor. The cavernoma was removed with ultrasonic aspirator (Integra CUSA, USA). Some part of the lesion was adherent to the walls of the third ventricle, and it was dissected with curettes. Multilayer “sandwich” repair of the skull-base defect was performed with fascia lata flap, autologous bone graft, abdominal fat graft, and fibrin-thrombin glue. Lumbar drain was placed at the end of the procedure.
Five patients were included into this study. There were four men and one woman. Mean age comprised 33.8 years (range 20-48 years). Most patients were admitted to our hospital due to visual disturbances (80%). Abrupt visual loss was observed in three patients, and gradient - in one patient (Table 1).
Patient #1 had features of diabetes insipidus caused by mass-effect. He was admitted to our hospital due to constant headaches. They were caused by the occlusion of the third and the left lateral ventricles by the large sellar lesion (Fig. 2). Patient #2 was investigated due to progressive visual loss and right-sided homonymous hemianopia. Patient #3 suffered abrupt left-sided visual loss and bitemporal hemianopia. Multiple cerebral cavernomas were revealed on MRI. One of them located in the superior medial chiasm. Patient #4 presented with severe visual disturbances and right-sided homonymous hemianopia (Figs. 3, 4). Concomitant left-sided ophthalmic ICA aneurysm was revealed on MRI which was clipped during the same procedure. Patient #5 suffered from decreased visual acuity and left-sided inferior nasal quadrantanopia. Hematoma related to hemorrhage from a cavernoma was observed in 3 patients.
Visual function improved in patients #2-5. Visual function was unchanged in patient #1 lacking visual disturbances pre-op. Significant improvement was registered in patient #2 (visual acuity increased from 0.20 to 1.00) who had presented with severe visual dysfunction initially (left-sided amaurosis, right-sided temporal hemianopia). Visual fields extended and visual acuity increased from 0.20 to 0.30 post-op in patient #3. In patient #4 minimal increase of visual acuity (from 0.03 to 0.08) and extension of the visual field of the right eye were registered. In patient #5 visual acuity increased from 0.30 to 0.50, and the visual field of the left eye extended in the temporal half.
Complication developed in one patient after endoscopic transnasal removal. Syndrome of transected pituitary stalk occurred with subsequent hypofunction of the pituitary gland and diabetes insipidus. It resolved under appropriate hormone substitution. However, one month later patient was readmitted to our hospital due to nasal cerebro spinal fluid (CSF)-leak and meningitis. The patient was re-operated, defect of the skull base was identified and repaired. After appropriate antibacterial therapy and lumbar drainage, the CSF-leak resolved and the patient was discharged.
Optochiasmatic cavernomas are extremely rare (less than 1% of all cavernomas) [13,17-19]. Since 1958 only 118 cases have been reported [4,21-23]. They are found in different parts of the optic nerves and optic chiasm, which determines the surgical strategy (either microsurgical, or endoscopic transnasal).
Optochiasmatic cavernomas have to be differentiated from craniopharyngiomas, gliomas, meningiomas, thrombosed aneurysms, schwannomas, etc [21-23]. Optic nerve glioma causes enlargement of the optic nerve or chiasm. However, no features of local hemorrhage are found [17]. Contrast enhancement is typical for optic gliomas.
Craniopharyngiomas are contrast-enhancing lesions that might have cystic and/or solid structure with calicifications [1,10]. Optic nerve sheath meningiomas usually have linear pattern of contrast enhancement along the optic nerve. CT-angiography, MR 3D-TOF angiography, or digital subtraction angiography are used to differentiate cavernomas from arterio-venous malformations and aneurysms. Pituitary apoplexy might also lead to chiasmatic hemorrhage; however, oculomotor dysfunction is usually observed in these patients due to compression of the cavernous sinus [2,16]. Optic neuritis can cause abrupt visual loss, and intensive contrast enhancement of the optic nerve can be recognized on MRI [7].
Optochiasmatic cavernomas typically present with sudden decrease of visual acuity, reduced visual fields, visual hallucinations [4,21]. Acute headache, retroorbital pain, nausea, and vomiting can develop as well [21-23]. Loss of consciousness and endocrinopathy might be observed in case of massive hemorrhage and compression of the hypothalamo-hypophyseal region [21,23].
MRI is a first-line diagnostic option in optochiasmatic cavernomas. Precise radiographic evaluation of the sellar region is mandatory. Optochiasmatic cavernomas usually appear as local bulging of the optic nerve or chiasm with a distinct signal intensity. They have iso- or hypointensive signal on T1WI. On T2WI cavernomas have heterogeneous pattern due to hemorrhagic component. Heme has signal of different intensity depending on a stage of its metabolism. Hence, this creates a characteristic pattern of “popcorn”. Surrounding hypointensive ring is caused by hemosiderin and is found in 60% of cases. Other MRI sequences include 3D Spoiled Gradient Recalled echo (SPGR), T2 CUBE, Fluid Attenuated Inversion Recovery (FLAIR), Susceptibility Weighted Angiography (SWAN) etc. Their use is obligatory in case of complicated differential diagnosis. Cavernomas are typically non-enhancing lesions; however, in certain cases contrast enhancement can be observed as well [3,21].
Surgical approach is one of the first issues that should be considered when treating optochiasmatic cavernomas. Cavernomas of the lateral parts of the optic chiasm and nerve as well as those located in the posterior half of the chiasm are amenable for microsurgical removal. The radiographic method of two points can be applied to determine whether microsurgery is appropriate option [4,6]. Cavernomas of the intracanal portion of the optic nerve and those located at its superior aspect are another indication for microsurgery. Anterior clinoidectomy should be performed in this case [4,12].
Pterional craniotomy is preferred option for these cavernomas. Additional osteotomy can be performed for more basal approach: orbitotomy, zygomatomy, anterior clinoidectomy [4,12]. Anterior interhemispheric transbasal approach has been also used for these lesions [19].
After meticulous arachnoid dissection and exposure of the optic apparatus are made, cavernoma-related hematoma is incised and evacuated. Then cavernoma is debulked using microscissors and mictodissectors.
Bipolar coagulation is usually avoided. However, low-voltage coagulation is used in certain cases [4]. It is accepted by many authors, that adjacent post-hemorrhagic gliotic tissue of the optic nerve and the optic chiasm should not be resected as this is not the part of cavernoma. Removal of this tissue can result into further visual decline [12].
Indocyanine green (ICG) videoangiography can be applied to increase intraoperative visualization. ICG enhancement of cavernoma develops within 1-2 minutes after intravenous injection and facilitates its localization [4,14].
Microsurgery has certain advantages over endoscopy, including better visualization of optic structures, especially anterior lateral portions of the optic nerves, chiasm and tracts [3,19]. However, it gives only limited access to the ventromedial portions of the optic apparatus [4].
Due to rarity of the disease, clear diagnosis is often challenging. Optochiasmatic cavernomas can be confused with craniophatyngiomas, gliomas and pituitary adenomas. These lesions are often removed via the anterior extended transsphenoidal approach [15]. Al-Sharydah et al. reported on case of transsphenoidal removal of the endo-suprasellar cavernoma, which was mimicking pituitary adenoma on pre-op MRI [3]. Some authors consider that endoscopic transsphenoidal removal is associated with worse outcomes due to inadequate decompression of the optic chiasm [12].
One of the main advantages of the transsphenoidal route is better visualization of the parasellar structures: the pituitary stalk, anterior and ventral parts of the chiasm and the optic nerves, the anterior communicating artery (AComA) complex. It allows to decrease traction injury and preserve perforating arteries. Hypophyseal arteries are easily visualized through the endoscopic approach, and therefore, the risk of their injury is diminished. Moreover, the transsphenoidal route provides the panoramic view under different angles which allows to overcome limitations of the unilateral subfrontal approach [15]. It should be noted that the main disadvantage of the transsphenoidal approach is the high risk of nasal CSF-leak and postoperative meningitis.
One patient in our series had large cavernoma extending to the third ventricle. Safe and complete removal became possible only after the pituitary stalk transection. It provided surgeons with better access to the retrochiasmatic region. The pituitary stalk craniopharyngioma was the initial diagnosis considered in this patient, and transection of the pituitary stalk was planned to remove the infundibulum and achieve total resection of the tumor. In our opinion the endoscopic approach can be safely applied in medially and inferiorly located optochiasmatic cavernomas, and those located in the optic canal. Otherwise, the transsphenoidal route is associated with high risk of injury to the optic apparatus and pituitary complex [22].
The optic structures can be injured during surgery either directly, or due to excessive traction and vascular damage. Pupillary diameter monitoring was suggested for avoidance of these complications [10]. Mydriasis indicates injury of the afferent visual fibers, and thus, dictates withdrawal of the further removal.
Direct stimulation of the optic nerves, tracts and chiasm is relatively new option. It is also used for other optochiasmatic lesions [4-6]. In case magnitudes of P20 and N30 waves start to decrease, surgeon is recommended to pause the procedure or withdraw further resection.
Visual functions improve after surgery in most patients with optochiasmatic cavernomas, according to our data and other studies (Table 2). It depends on the pre-op visual status of the patient: visual acuity usually increases in 50-75% of the cases. Extension of visual fields is observed as well. Improvement usually develops over time and can be discovered at the follow-up examination and automatic perimetry. It should be noted that the risk of further visual decline is greater in conservatively treated patients. Moreover, the extent of resection (total or subtotal) is reported not to influence visual outcomes significantly [1,2,8,11,23]. Hence, surgical treatment in patients with impaired visual function can provide better visual outcomes with relatively low risk of complications.
Optochiasmatic cavernomas are encountered extremely rare. Despite the use of contemporary diagnostic options, differential diagnosis remains challenging. Full diagnostic work-up is mandatory. After the diagnosis is made, surgical treatment should be considered first. Total microsurgical or endoscopic transsphenoidal removal of the optochiasmatic cavernoma is relatively safe and effective treatment method facilitating improvement of visual function.
Fig. 1.
Schematic drawing demonstrating cavernoma location and applied surgical approach (microsurgical subfrontal and endoscopic transsphenoidal) in present series.
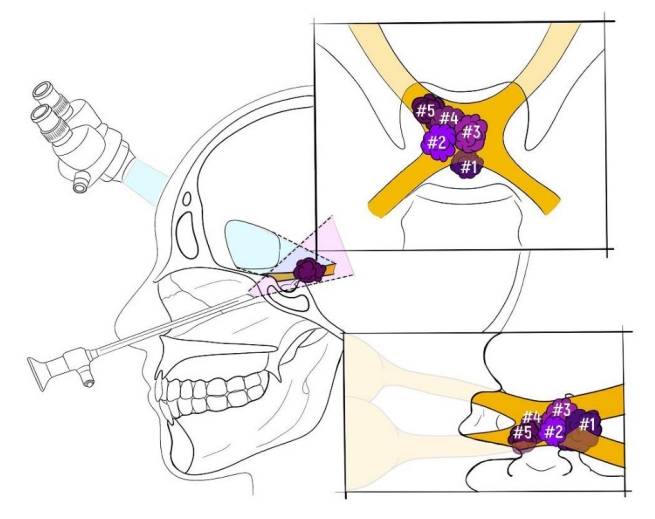
Fig. 2.
Clinical case of endoscopic transnasal resection of the cavernoma of the chiasm and the third ventricle. (A) Chiasmatic lesion (arrow) extending to the third ventricle is shown on frontal T1-weighted contrast-enhanced images, (B) heterogeneous lesion (arrow) with post-hemorrhagic features on sagittal T1-weighted contrast-enhanced images, (C) CT scan 1 day post-op showing complete removal, (D) intraoperative endoscopic view, post-resection cavity and hematoma are depicted (arrow). CT, computed tomography; PComA, posterior communicating artery aneurysm
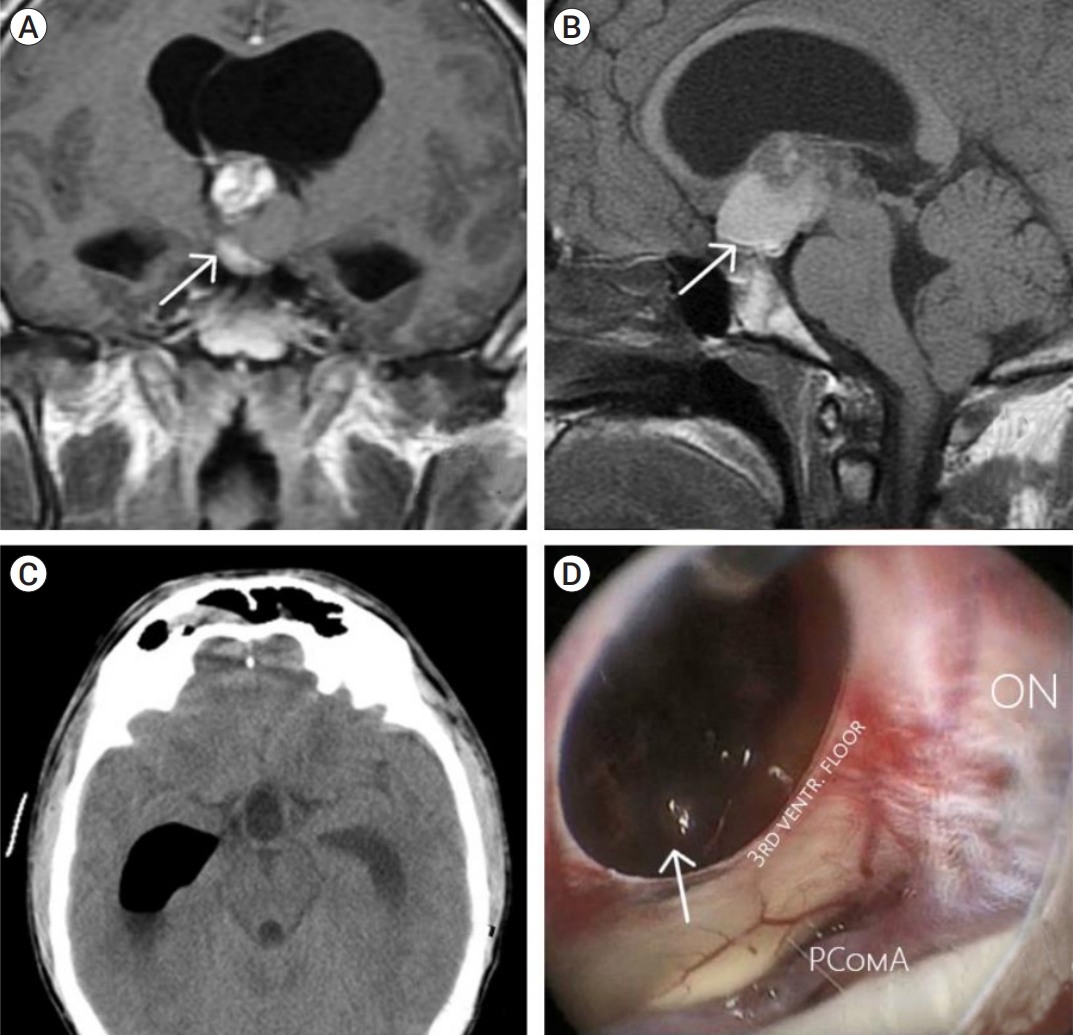
Fig. 3.
Clinical case of microsurgical removal of a cavernoma of the left optic nerve. (A) Hypointentsive bulging of the left optic nerve (arrow) is visible on axial slices, T2WI, FIESTA, (B) heterogeneous, mostly isointensive zone in the region of the left optic nerve (arrow) on frontal slices, T2WI, (C) intraoperative view of a left-sided subfrontal approach, cavernoma (arrow) is easily recognizable in the left optic nerve, (D) post-resection cavity.
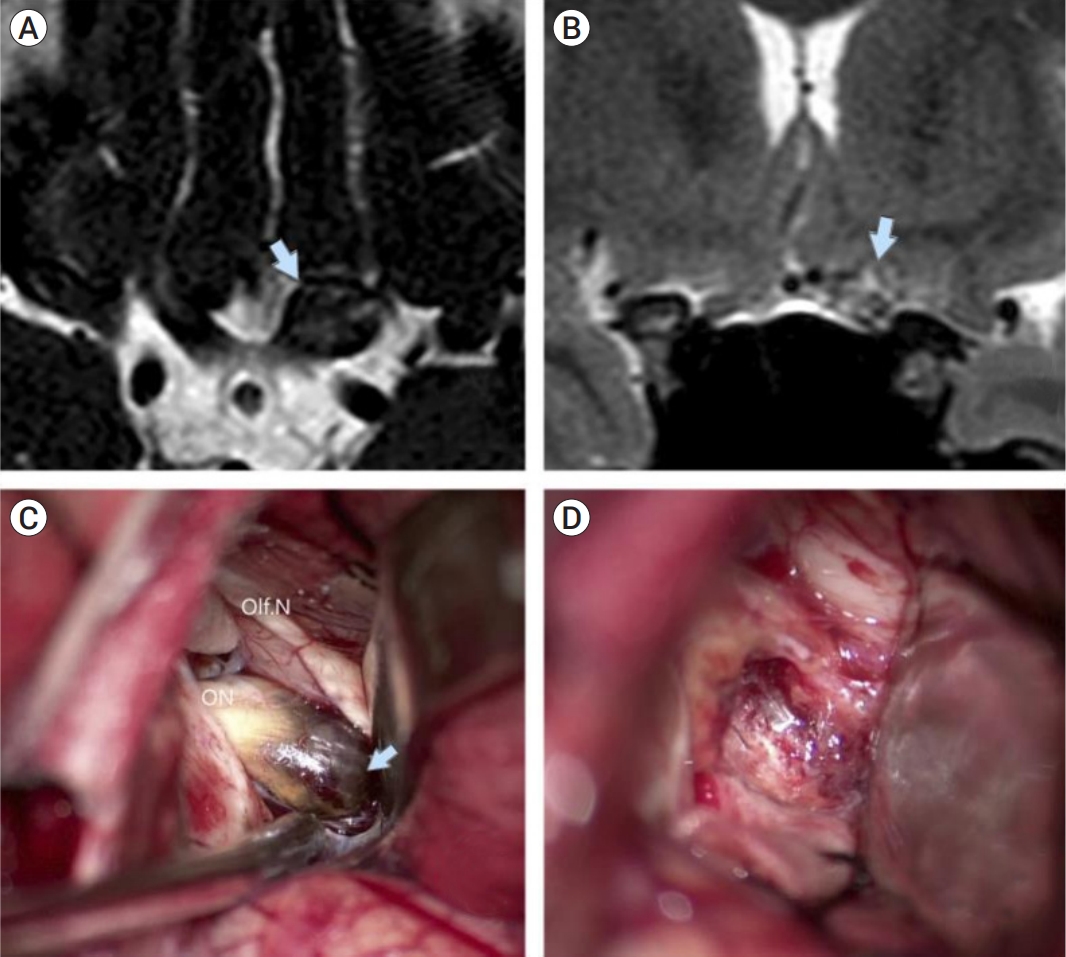
Fig. 4.
Visual fields of a patient number 4. (A) Pre-op automatic perimetry showing a deficit in the lower part of the visual field of the right eye, (B) perimetry showing slight improvement of the visual field one-month post-op.
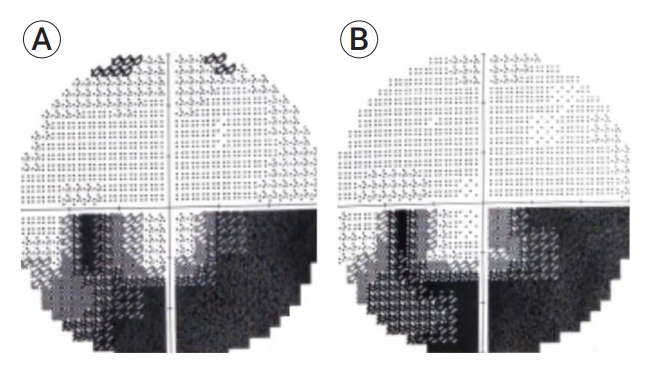
Table 1.
Clinical and surgical data of the series
Table 2.
Comparative analysis of surgical series of optochiasmatic cavernomas
REFERENCES
1. Alam S, Chaurasia BK, Shalike N, Uddin AN, Chowdhury D, Khan AH, et al. Surgical management of clinoidal meningiomas: 10 cases analysis. Neuroimmunology and Neuroinflammation. 2018 May;5:21.

2. Alam S, Ferini G, Muhammad N, Ahmed N, Wakil AN, Islam KM, et al. Skull base approaches for tuberculum sellae meningiomas: Institutional experience in a series of 34 patients. Life (Basel). 2022 Mar;12(4):492.



3. Algoet M, Van Dyck-Lippens PJ, Casselman J, Sirimsi S, Fletcher CDM, Van Den Berghe I, et al. Intracanal optic nerve cavernous hemangioma: A case report and review of the literature. World Neurosurg. 2019 Jun;126:428-33.


4. Benedičič M, Bošnjak R. Intraoperative monitoring of the visual function using cortical potentials after electrical epidural stimulation of the optic nerve. Acta Neurochir (Wien). 2011 Oct;153(10):1919-27.


5. Bosnjak R, Benedicic M. Direct epidural electrical stimulation of the optic nerve: A new method for intraoperative assessment of function. J Neurosurg. 2008 Oct;109(4):647-53.


6. Brown AP, Thompson BG, Spetzler RF. The two-point method: Evaluating brain stem lesions. Barrow Q. 1996 12:20-4.
7. Cerase A, Franceschini R, Battistini S, Maria Vallone I, Penco S, Venturi C. Cavernous malformation of the optic nerve mimicking optic neuritis. J Neuroophthalmol. 2010 Jun;30(2):126-31.


8. Chaurasia B. Extended endonasal endoscopic approach for the resection of craniopharyngioma-an analysis of 40 cases. EC Neurology. 2018 10:981-90.
9. Chen Y, Tu Y, Chen B, Shi J, Yu B, Wu W. Endoscopic transnasal removal of cavernous hemangiomas of the optic canal. Am J Ophthalmol. 2017 Jan;173:1-6.


11. Garg K, Chaurasia B, Pahwa B, Arnaout MM, Zenonos GA, Piloto OL, et al. Geographic Disparities in the proliferation of minimally invasive approaches for sellar/parasellar lesions. World Neurosurgery. 2022 Dec;168:e162-77.

12. Gonçalves VM, Gonçalves V. Surgical management of cavernous malformation of the optic nerve with canalicular extension. Surg Neurol Int. 2014 Oct;5(Suppl 12):S455-60.


13. Meng X, Feng X, Wan J. Endoscopic endonasal transsphenoidal approach for the removal of optochiasmatic cavernoma: Case report and literature review. World Neurosurg. 2017 Oct;106:1053.

14. Murai Y, Adachi K, Koketsu K, Teramoto A. Indocyanine green videoangiography of optic cavernous angioma -case report-. Neurol Med Chir (Tokyo). 2011 51(4):296-8.


15. Muta D, Nishi T, Koga K, Yamashiro S, Fujioka S, Kuratsu J. Cavernous malformation of the optic chiasm: Case report. Br J Neurosurg. 2006 Oct;20(5):312-5.


16. Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy: A review. J Intensive Care Med. 2008 Mar-Apr;23(2):75-90.


17. Ozer E, Kalemci O, Yücesoy K, Canda S. Optochiasmatic cavernous angioma: Unexpected diagnosis. Case report. Neurol Med Chir (Tokyo). 2007 Mar;47(3):128-31.


18. San-Juan D, Escanio Cortés M, Tena-Suck M, Orozco Garduño AJ, López Pizano JA, Villanueva Domínguez J, et al. Neurophysiological intraoperative monitoring during an optic nerve schwannoma removal. J Clin Monit Comput. 2017 Oct;31(5):1059-64.


19. Sbeih I, Darwazeh R, Shehadeh M, Nisah M, Sbeih A, Abu-Farsakh H, et al. Anterior interhemispheric approach for microsurgical resection of an optic chiasm cavernoma. Interdiscip Neurosurg. 2020 21:100766.

20. Srinivasan VM, Koester SW, Wang MS, Rahmani R, Ma KL, Catapano JS, et al. Cavernous malformations of the optic nerve and optic pathway: A case series and systematic review of the literature. Oper Neurosurg (Hagerstown). 2021 Oct;21(5):291-302.


21. Tan T, Tee JW, Trost N, McKelvie P, Wang YY. Anterior visual pathway cavernous malformations. J Clin Neurosci. 2015 Feb;22(2):258-67.


- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 1,836 View
- 26 Download
- ORCID iDs
-
Bipin Chaurasia

https://orcid.org/0000-0002-8392-2072 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print