 |
 |
| J Cerebrovasc Endovasc Neurosurg > Epub ahead of print |
Abstract
Isolated middle cerebral artery dissection (MCAD) is rare but increasingly recognized as a significant clinical entity, particularly in younger adults. Ischemic stroke is the most common manifestation in symptomatic cases but symptoms can vary in severity from headaches to severe neurologic deficits. Due to its rarity and unpredictable clinical course, there is no established treatment strategy for isolated MCAD. Through two case reports, we reviewed the post-operative clinical course of MCAD under different treatment modalities. Case 1 was a 21-year-old woman who presented to the emergency department with headaches and left-side hemiparesis. Isolated MCAD was diagnosed and she was successfully treated with the placement of a self-expandable stent and subsequent chemical angioplasty for post-stent vasospasm. Case 2 was a 35-year-old woman who presented to the emergency department with left-side hemiparesis and dysarthria. Isolated MCAD was diagnosed and she was successfully treated with superficial temporal artery (STA) to middle cerebral artery (MCA) anastomosis.
Cervicocephalic artery dissection (CCAD), representing an intramural hematoma of a cervical or an intracranial artery, is the major cause of acute ischemic stroke (AIS) in children and young adults [1,18]. In contrast to extracranial artery dissection (EAD), which has been studied extensively, less is known about intracranial artery dissection (IAD) [5,12]. The clinical presentation of IAD varies. The two main manifestations are subarachnoid hemorrhage (SAH) and cerebral ischemia [4]. No randomized trials have yet been conducted so there are no standardized guidelines on the treatment of patients with IAD. Among IADs, which the gold standard treatment method has not been established, we report two cases of spontaneous, isolated MCAD presenting as AIS without SAH treated with different methods endovascular stenting and bypass surgery.
A 21-year-old, right-handed woman was transferred to the emergency department with a headache and left-sided face, arm and leg weakness that had begun 1 day earlier. She had no past medical or trauma history. Magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) showed acute infarctions in the right middle cerebral artery (MCA) territory (Fig. 1A) with diffuse, severe narrowing of the MCA and occlusion of the distal internal carotid artery (ICA). The MRI/MRA also revealed the ŌĆ£double-lumen signŌĆØ (Fig. 1B). The patient was administered a loading dose of aspirin (500 mg) and clopidogrel (300 mg) and then started on oral aspirin (100 mg/d) and clopidogrel (75 mg/d). The results of initial transfemoral cerebral angiography (TFCA) were compatible with those of the MRI/MRA. The image findings and her young age strongly suggested a diagnosis of IAD. After the TFCA, we continued treating the patient with the dual antiplatelet agents indicated above and her left-side motor strength improved slightly from 4/4 to 4+/4+ (upper/lower).
On day 3 of hospitalization, additional TFCA and intervention were requested because the patientŌĆÖs left upper and lower extremity strength worsened to 1/3. The angiogram showed near occlusion of the right distal ICA with severe and persisting flow limitation to the right hemisphere (Fig. 2A).
After puncture of the right femoral artery, we inserted an 8-F sheath shuttle, which was placed into the right ICA cavernous segment. Then, a microcatheter (Prowler select plus microcatheter; Cerenovus, Johnson & Johnson Medical Devices, Irvine, California, USA) was navigated carefully into the segment of dissected MCA over a 0.010-inch microwire (Synchro-10; Stryker, Fremont, California, USA). A self-expandable, closedcell intracranial stent (Enterprise stent; Cerenovus, Johnson & Johnson Medical Devices, Irvine, California, USA) was successfully positioned at the area of the dissection and deployed across the dissection. The flow to the right hemisphere showed improvement in a poststent placement angiogram (Fig. 2B). After the procedure, the patient was started on continuous tirofiban (0.1 mcg/kg/min) and argatroban (2.5 mg/h) for 3 days. Her left-side motor strength improved to 4/4.
The patient was discharged from the hospital with no motor weakness on day 30 of hospitalization. At a 6-month post-procedure follow-up, a TFCA showed no in-stent or adjacent-stent stenosis and near-normalization of the size of the parent vessel (Fig. 3). The patientŌĆÖs motor strength was 5/5 and there were no focal neurologic deficits.
A 35-year-old, right-handed woman was transferred to the emergency department for left-side hemiparesis and dysarthria 8 hours after symptom onset. She had no past medical or trauma history. An MRI/MRA scan showed acute infarctions in the right MCA territory (Fig. 4A) with no evidence of subarachnoid hemorrhage. A TFCA revealed acute proximal M1 occlusion (Fig. 4B). As in case 1, the patient was initially started on dual antiplatelet agents (aspirin and clopidogrel). Because of ŌĆ£double-lumen signŌĆØ on her MRI/MRA, the patientŌĆÖs young age and fluctuating clinical course, a diagnosis of middle cerebral artery dissection (MCAD) could be suspected. Conservative treatment was selected first because the clinical symptoms were mild compared to the radiologic finding, which seemed very risky for endovascular treatment.
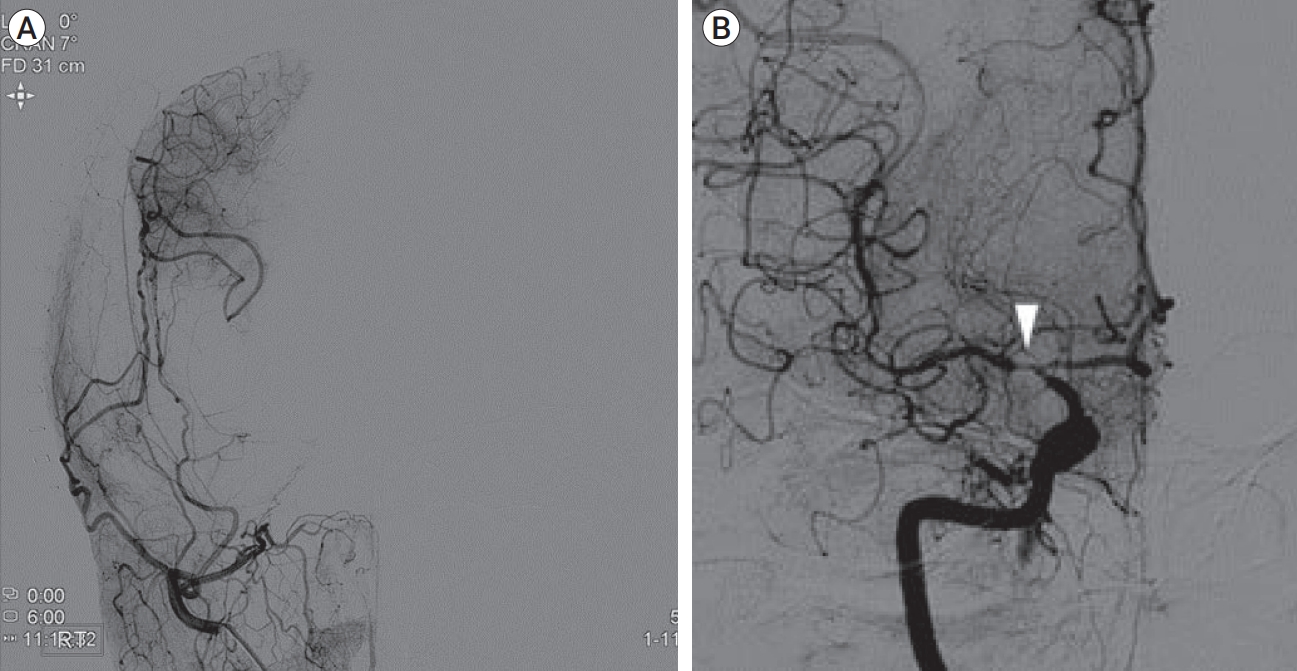
But, on day 3 of hospitalization, the patientŌĆÖs left-side motor strength deteriorated from 4/4 to 2/2. We decided that urgent superficial temporal artery to middle cerebral artery (STA-MCA) bypass surgery would be a better treatment option than endovascular treatment (EVT) considering risk of complication according to different treatment modalities. End-to-side anastomosis was performed between the parietal branch of the right STA and the M4 segment of the MCA. A TFCA on postoperative day 5 showed good patency of the graft to the M4 segment (Fig. 5A) and retrograde recanalization of the right proximal M1 (Fig. 5B). The relatively short time in which MCA patency was recovered through retrograde flow via the bypass graft provided stronger confirmation of our MCAD diagnosis.
The patientŌĆÖs postoperative course was stable and uneventful, with no further cerebral infarction. She was discharged to a rehabilitation hospital with left-side hemiparesis (motor grade 2/2) on postoperative day 14 and continued the dual antiplatelet agent treatment after discharge. At her 6-month follow-up, the patientŌĆÖs left-side motor strength had improved from motor grade 2/2 to 4/4.
Isolated IAD is an uncommon cause of stroke [3]. The symptoms vary but its two main manifestations are SAH and cerebral ischemia [13]. Between 30% and 78% of patients with IAD present with cerebral ischemia without SAH. The underlying stroke mechanism could be hemodynamic, thromboembolic or occlusion of a perforating artery by the mural hematoma [4].
Treatment guidelines for this condition are not well-established owing to a lack of randomized trials and only observational studies with small sample sizes [4]. In 2021, the European Stroke Organization (ESO) published suggested guidelines for the management of extracranial and intracranial artery dissection [6]. The guidelines provide one evidence-based recommendation and three expert consensus statements regarding the management of IAD. In brief, the guidelines are as follows.
Within 4.5 hours of onset and after ruling out standard contraindications, including subtle signs of subarachnoid bleeding on brain imaging, intravenous thrombolysis (IVT) with alteplase may initially be considered in patients with symptomatic IAD with AIS. Given the risk of SAH and the superiority of aspirin over anticoagulants in the acute phase of ischemic stroke, antiplatelet agents have a better risk/benefit ratio than anticoagulants in patients with symptomatic IAD with AIS. EVT for the treatment of the IAD lesions is suggested in AIS with IAD and large vessel occlusion. Early surgical or endovascular intervention is required in patients with IAD and SAH.
The ESO guidelines have some limitations. The guidelines suggest that medical treatment is preferable to active EVT in patients with AIS symptoms unless there is complete large vessel occlusion. However, if EVT is delayed until symptoms and lesions worsen, the procedure becomes more difficult and the prognosis may be worse due to advanced lesions. In case 1, above, we believe that EVT could have yielded better clinical outcomes if it had been performed before the lesion became completely occluded. Furthermore, the guidelines make no mention of bypass surgery as a treatment option. In case 2 of this report, urgent bypass surgery was performed due to progressive neurological deterioration and good results were obtained.
In our two cases, IVT with alteplase was not considered in either of our two cases because more than 4.5 hours had passed since stroke onset when they presented to the emergency department. The patients were loaded with dual antiplatelet agents (aspirin and clopidogrel) after AIS with isolated MCAD was postulated. Despite this initial medical management, their left-side motor weakness was aggravated. Therefore, we performed EVT with stenting for the MCAD lesion in case 1, and urgent STA-MCA anastomosis in case 2.
In case 1, we used a self-expanding stent, followed by intravenous tirofiban (IV antiplatelet) infusion. Kim et al. have reported eight IADs treated with self-expanding stent placement, with 3-month modified Rankin Scale (mRS) scores of 0-2 in all eight [9]. All their stents were patent and there was no significant residual intra-stent stenosis at angiographic follow-up. Relative to other types, self-expanding stents are easy to deliver and have low radial force, making them a safe and sufficient option for expansion of a collapsed lumen as dissections tend to be compliant and rarely have atheromatous or calcified plaques [9,11]. Bernava et al. reported seven IAD cases treated with self-expanding stents and concomitant tirofiban administration [2]. In their study, mid-term follow-ups showed parent artery patency in 6/7 cases (85.7%), with mRS scores of Ōēż0-2 at 3 months in 5/7 cases (71.4%) [2]. In our stent-treated case, both the flow to the right cerebral hemisphere and left-side motor strength initially improved immediately after EVT with stenting and concomitant tirofiban. However, the day after the procedure, the patientŌĆÖs motor weakness fluctuated. Follow-up TFCA revealed in-stent thrombosis, so intra-arterial tissue plasminogen activator (tPA) injection was performed. The thrombolysis was successful and IV argatroban (direct thrombin inhibitor) was administered in addition to the tirofiban. After that, the patient showed a stable clinical course. Jang et al. suggest that concomitant treatment with argatroban and glycoprotein IIb/IIIa inhibitors are well-tolerated and can provide adequate anticoagulation with an acceptable bleeding risk for patients undergoing percutaneous coronary intervention [8]. In our case, tirofiban with argatroban anticoagulation was found to be a safe and effective means of preventing in-stent thrombosis. Unfortunately, the patientŌĆÖs left-side motor weakness deteriorated again the day after thrombolysis. Follow-up TFCA revealed post-stent stenosis. To our knowledge, no other reports have described post-stent stenosis or vasospasm after stenting for IAD presenting with ischemia. In our case, the post-stent stenosis was ameliorated by intra-arterial injection of verapamil, suggesting that post-stent vasospasm was the most likely cause. After this, the patientŌĆÖs left upper extremity strength improved to 4/4 and she was started on nimodipine (180 mg/d) to prevent vasospasm.
Our experience suggests that vasospasm should be considered when ischemia recurs after stenting for IAD. We found intra-arterial vasodilator injection to be an effective treatment for vasospasm.
In case 2, urgent STA-MCA anastomosis was performed 3 days after symptom onset to prevent further cerebral infarction. There are a few case reports of IAD treated with STA-MCA anastomosis and the indications for surgical intervention are not yet firmly established [7,10,14-17]. Oka et al. classified patients with IAD presenting as AIS into three types: (A) Single attack with severe outcomes (cannot walk); (B) Single attack with mild to moderate outcomes (can walk); (C) Recurrent attacks with various outcomes. They propose that STA-MCA anastomosis is indicated for type C patients [15]. In our case, the patient was type C and the bypass surgery prevented further attacks. Ikota et al. have reported a case of AIS due to IAD successfully treated with STA-MCA anastomosis. They state that early bypass surgery could salvage the cortical penumbra in the acute phase. In their case, TFCA 6 months after the surgery revealed spontaneous resolution of the dissection [7]. In the present case, TFCA on postoperative day 5 showed partial recanalization of the dissection segment, suggesting that, with the concomitant use of antiplatelet agents, retrograde blood flow might stabilize the dissection flap. The patient recovered well without further ischaemic attacks and her left-side hemiparesis had improved significantly from motor grade 2/2 to 4/4 6 months after the surgery.
Both endovascular stenting and STA-MCA anastomosis can be good treatment options for aggravating AIS due to IAD. Endovascular stenting is a shorter less invasive procedure than bypass surgery but carries a risk of arterial rupture if a microcatheter passes through the pseudo-lumen and arterial expansion is performed after stent deployment. Bypass surgery is a longer, more complex operation and requires experience and skill in the necessary neurosurgical techniques. It carries a risk of postoperative hemorrhagic complications due to the prior use of antiplatelet agents. However, since retrograde flow through the bypass graft in the opposite direction to dissection progression can stabilize the dissection flap, it is safer in terms of avoidance of lesion perforation.
Thus, we recommend multi-modal treatment options for IAD presenting as AIS with individualized treatment selection based on patient suitability, intervention timing and the benefits and pitfalls of each option.
As a kind of IAD, isolated MCAD with AIS is rare. We have presented the two cases of successful treatment for isolated MCAD presenting as AIS based on either endovascular self-expanding stent placement or STA-MCA anastomosis with concomitant use of antiplatelet agents. Multi-modal treatment options should be considered in aggravating AIS with isolated MCAD.
ACKNOWLEDGEMENTS
We are indebted to the neuroradiologists in our institute, who are always cooperative with us in the treatment of complex neurosurgical diseases.
Fig.┬Ā1.
(A) A diffusion-weighted MRI showing acute infarctions in the right MCA territory; (B) TOF-MRA shows severe and diffuse narrowing of the M1 segment of the MCA and occlusion of the distal ICA. In the MRA, the ŌĆ£double-lumen signŌĆØ can be seen on the M1 segment of the MCA (white arrowhead). MRI, magnetic resonance imaging; MCA, middle cerebral artery; TOF-MRA, time-of-flight magnetic resonance angiography; ICA, internal carotid artery; MRA, magnetic resonance angiography
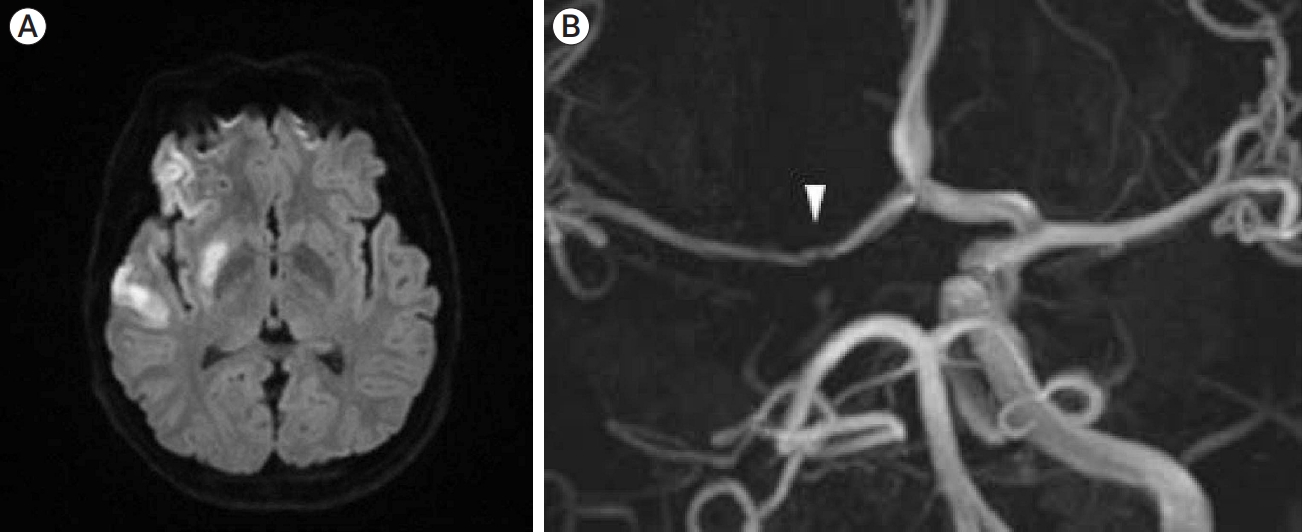
Fig.┬Ā2.
(A) A follow-up TFCA performed on day 3 of hospitalization showing long-segment severe, irregular stenosis from the right distal ICA, just distal to the ophthalmic segment, to the M1 segment of the MCA, with symptom aggravation. Aggravated flow limitation to the right hemisphere was due to the progressive dissection of the M1 segment of the MCA which cause a decrease in collateral flow via the Acom A; (B) A post-stent angiogram (AP view) showing improved blood flow to the right hemisphere and the restoration of ICA and MCA flow. However, ACA flow cannot be seen. The patient was medicated and kept under close observation without further intervention as the patency of the Acom A and contralateral A1 segment of the ACA were good. The proximal and distal ends of the stent were marked with white arrowheads. TFCA, transfemoral cerebral angiography; ICA, internal carotid artery; MCA, middle cerebral artery; Acom A, anterior communication artery; AP, anterior to posterior; ACA, anterior cerebral artery

Fig.┬Ā3.
A follow-up TFCA performed 6 months after the procedure showing no in-stent or stent-adjacent stenosis. There is near-normalization of the blood flow, including that from the ACA to the right hemisphere (AP view). TFCA, transfemoral cerebral angiography; ACA, anterior cerebral artery; AP, anterior to posterior
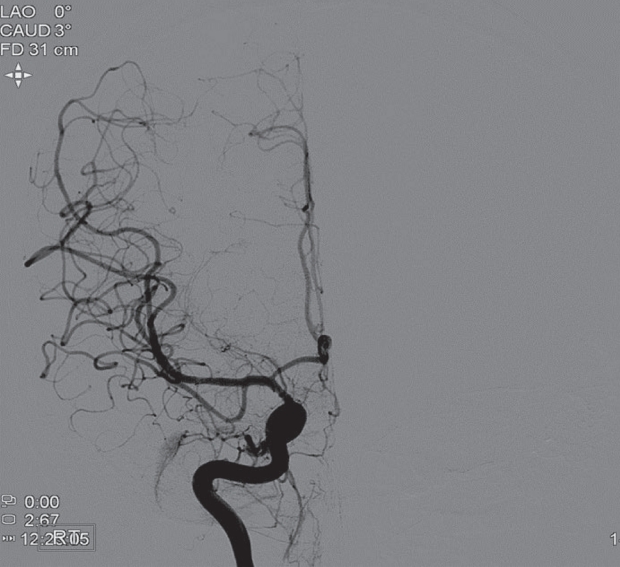
Fig.┬Ā4.
(A) A diffusion-weighted MRI showing acute infarctions in the right MCA territory; (B) An initial TFCA showing acute proximal occlusion of the M1 segment of the MCA with no basal collateral flow (AP view). MRI, magnetic resonance imaging; MCA, middle cerebral artery; TFCA, transfemoral cerebral angiography; AP, anterior to posterior

Fig.┬Ā5.
(A) A TFCA from postoperative day 5 showing good patency of the superficial temporal artery graft with the flow from the graft filling the M2 segment of the MCA in the right selective ECA angiogram; (B) A right selective ICA angiogram showing retrograde recanalization of the right M1 segment of the MCA with mild stenosis (white arrowhead) under the healing dissection (AP view). TFCA, transfemoral cerebral angiography; MCA, middle cerebral artery; ECA, external carotid artery; ICA, internal carotid artery; AP, anterior to posterior
REFERENCES
1. Amlie-Lefond C, Bernard TJ, Sebire G, Friedman NR, Heyer GL, Lerner NB, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: Results of the International Pediatric Stroke Study. Circulation. 2009 Mar;119(10):1417-23.



2. Bernava G, Meling TR, Rosi A, Hofmeister J, Yilmaz H, Brina O, et al. Acute stenting and concomitant tirofiban administration for the endovascular treatment of acute ischemic stroke related to intracranial artery dissections: A single center experience and systematic review of the literature. J Stroke Cerebrovasc Dis. 2021 Aug;30(8):105891.


4. Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015 Jun;14(6):640-54.


5. Debette S, Grond-Ginsbach C, Bodenant M, Kloss M, Engelter S, Metso T, et al. Differential features of carotid and vertebral artery dissections: The CADISP study. Neurology. 2011 Sep;77(12):1174-81.


6. Debette S, Mazighi M, Bijlenga P, Pezzini A, Koga M, Bersano A, et al. ESO guideline for the management of extracranial and intracranial artery dissection. Eur Stroke J. 2021 Sep;6(3):XXXIX-LXXXVIII.




7. Ikota M, Kusaka G, Tanaka Y. Superficial temporal artery-middle cerebral artery anastomosis for ischemic stroke due to dissection of the intracranial internal carotid artery with middle cerebral artery extension. NMC Case Rep J. 2018 Mar;5(2):39-44.



8. Jang IK, Lewis BE, Matthai WH Jr, Kleiman NS. Argatroban anticoagulation in conjunction with glycoprotein IIb/IIIa inhibition in patients undergoing percutaneous coronary intervention: An open-label, nonrandomized pilot study. J Thromb Thrombolysis. 2004 Aug;18(1):31-7.



9. Kim DJ, Kim BM, Suh SH, Kim DI. Self-expanding stent placement for anterior circulation intracranial artery dissection presenting with ischemic symptoms. Neurosurgery. 2015 Feb;76(2):158-64; discussion 64.



10. Kitani R, Itouji T, Noda Y, Kimura M, Uchida S. Dissecting aneurysms of the anterior circle of Willis arteries. Report of two cases. J Neurosurg. 1987 Aug;67(2):296-300.

11. Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Smith WS, et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol. 2000 Aug;21(7):1280-92.


12. Markus HS, Levi C, King A, Madigan J, Norris J. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: The Cervical Artery Dissection in Stroke Study (CADISS) randomized clinical trial final results. JAMA Neurol. 2019 Jun;76(6):657-64.



13. Metso TM, Metso AJ, Helenius J, Haapaniemi E, Salonen O, Porras M, et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke. 2007 Jun;38(6):1837-42.


14. Ogiwara H, Maeda K, Hara T, Kimura T, Abe H. Spontaneous intracranial internal carotid artery dissection treated by intra-arterial thrombolysis and superficial temporal artery-middle cerebral artery anastomosis in the acute stage-- Case report. Neurol Med Chir (Tokyo). 2005 Mar;45(3):148-51.


15. Oka F, Shimizu H, Matsumoto Y, Watanabe M, Tominaga T. Ischemic stroke due to dissection of intracranial internal carotid artery: Implications for early surgical treatment. Surg Neurol. 2008 Jun;69(6):578-84; discussion 584-5.


16. Ono H, Inoue T, Suematsu S, Tanishima T, Tamura A, Saito I, et al. Middle cerebral artery dissection causing subarachnoid hemorrhage and cerebral infarction: Trapping with high-flow bypass preserving the lenticulostriate artery. Surg Neurol Int. 2017 Jul;8:157.



-
METRICS

-
- 0 Crossref
- 0 Scopus
- 626 View
- 21 Download
- ORCID iDs
-
Kuhyun Yang

https://orcid.org/0000-0003-0019-6122 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



