 |
 |
| J Cerebrovasc Endovasc Neurosurg > Epub ahead of print |
Abstract
Objective
Double microcatheter technique (dMC) can be the alternative to Single microcatheter technique (sMC) for challenging cases, but there is lack of studies comparing dMC to sMC especifically for small ruptured aneurysms. Our objective was to compare the safety and efficacy of dMC to sMC in treating small (≤5 mm) and tiny (≤3 mm) ruptured aneurysms.
Methods
This study focused on 91 out of 280 patients who had ruptured aneurysms and underwent either single or double microcatheter coil embolization. These patients were treated with either single or double microcatheter coil embolization. We divided the patients into two groups based on the procedural method and evaluated clinical features and outcomes. Subgroup analyses were conducted specifically for tiny aneurysms, comparing the two methods, and within the dMC group, we also examined whether the aneurysm was tiny or not. In addition, univariate logistic regression analysis was performed to assess the impact of coil packing density.
Results
The mean values for most outcome measures in the dMC group were higher than those in the sMC group, but these differences did not reach statistical significance (coil packing density, 45.739% vs. 39.943%; procedural complication, 4.17% vs. 11.94%; recanalization, 8.3% vs. 10.45%; discharge discharge modified Rankin Scale (mRS), 1.83 vs. 1.97). The comparison between tiny aneurysms and other sizes within the dMC group did not reveal any significant differences in terms of worse outcomes or increased risk. The only factor that significantly influenced coil packing density in the univariate logistic regression analysis was the size of the aneurysm (OR 0.309, 95% CI 0.169-0.566, p=0.000).
Non-traumatic subarachnoid hemorrhage, often resulting from the rupture of intracranial aneurysms, remains a critical and urgent medical condition [17]. While aneurysmal clipping was previously a widely used method for aneurysmal subarachnoid hemorrhage (aSAH), endovascular coil embolization has emerged as a preferred option, with its application expanding [16].
The conventional single microcatheter technique (sMC) has been the primary choice for treatment. However, when dealing with very small aneurysms, performing coil embolization using this technique can be challenging [5,6,16,18]. There is ongoing debate regarding the appropriate procedural method for endovascular treatment of ruptured small aneurysms.
Stent-assisted coil embolization may be considered as an alternative option [3,4,9,25], but it has limitations as a standalone approach. Many patients with ruptured aneurysms have not received continuous antiplatelet therapy, and even with preprocedural administration of antiplatelet agents, the risk of thrombotic complications cannot be completely eliminated [4]. Moreover, there is a possibility of requiring additional surgical procedures after the initial coil embolization, which poses a significant burden in terms of continuous antiplatelet agent use. Considering these factors, the dMC can be employed as a secondary option to the conventional sMC. The double microcatheter technique (dMC) involves the deployment of two coils to provide mutual support and stability, creating a secure coil frame, and has been reported for coil embolization of cerebral aneurysms with complex configurations [13]. To the best of our knowledge, there is a lack of comprehensive studies comparing the outcomes of small ruptured aneurysms treated with the dMC versus the sMC.
The objective of this study is to examine the safety and efficacy of the dMC compared to the sMC in treating small ruptured aneurysms. Furthermore, we aim to evaluate the safety of the dMC in tiny ruptured aneurysms compared to small ruptured aneurysms.
This retrospective study focused on patients who received endovascular treatment for aSAH at a single center. We accessed patient data from the center’s electronic database, resulting in the inclusion of 280 patients who underwent endovascular treatment for ruptured aneurysm between March 2011 and April 2022. For this study, only cases that met specific criteria were included: (1) true saccular aneurysms with a maximum diameter in any direction of 5 mm or less and (2) coil embolization using either sMC or dMC. Patients with (1) fusiform aneurysms, dissecting aneurysms, or blister aneurysms or (2) who received stent-assisted coil embolization, flow diverter insertion, or coil trapping were excluded. Finally, 91 patients were enrolled in the analysis.
The 91 cases were divided into two groups: dMC and sMC. We analyzed the demographic and clinical characteristics of each group and evaluated various outcome measures, including coil packing density, immediate Raymond-Roy occlusion classification (RROC), procedural complications, discharge modified Rankin Scale (mRS), in-hospital mortality, and recanalization. The study also included two subgroups. The first subgroup comprised patients with tiny aneurysms (≤3 mm), while the second subgroup consisted of patients who underwent dMC. The treatment approach and outcomes were analyzed for the tiny aneurysm subgroup similar to the overall study population. Additionally, the double microcatheter subgroup was analyzed to compare treatment outcomes that were not classified as tiny (>3 mm, ≦5 mm).
To identify factors strongly correlated with the outcomes, univariate logistic regression analysis was conducted on all outcome measures. For coil packing density, a density above 40% was defined as high packing density. Other outcome measures were refined to indicate favorable results, such as a RROC of 0, absence of procedural complications, no recanalization, a favorable mRS score of 0-1, and no mortality.
The study received approval from the institutional review board, allowing the review and publication of information obtained from patient records.
All patients were considered suitable candidates for endovascular coil embolization, as determined by a consensus among neurosurgeons and neurointerventionalists, unless patients refused surgery. The following process and strategies were employed for coil embolization:
1. The endovascular procedures were performed under general anesthesia using a commercially available monoplane angiography unit, with a common femoral approach.
2. Multiple imaging views, including 3D rotational angiography, were used to assess the characteristics of the aneurysm. This assessment included examining the height and width of the aneurysm dome and the size of the neck to determine the feasibility of deploying coils to form a stable basket.
3. A hemostatic valve with double ports was connected to the guiding catheter that contained the first microcatheter. The first microcatheter was positioned within the aneurysm sac, and the efforts were made to create a framework.
4. If the framework was adequately maintained, additional coils were inserted though the same microcatheter.
5. In cases where the frame coil herniated, a partial frame was formed using only a part of the first coil. Subsequently, an additional microcatheter was positioned within the aneurysm sac, and coil embolization was performed using the double catheter technique.
6. The coils were intertwined and provided mutual supported to form a frame basket. If the framing was unsatisfactory, one or both coils could be retrieved and reinserted separately.
7. The aneurysms were densely coiled until it was no longer possible to deploy additional coils. This process involved alternating detachment of one of the two coils and advancing the next coil.
The assessment of procedural outcomes focused on coil packing density and procedure complications. Coil packing density was determined by dividing the volume of the coils by the volume of the aneurysm, and the result was expressed as a percentage.
The volume of the aneurysm was calculated using the ellipsoid formula, which assumes that aneurysms have an ellipsoidal shape. The formula used was as follows:
Aneurysm volume = 4/3π(A/2)(B/2)(A+B)/4
In this formula, A and B represent the largest horizontal and vertical diameters of the aneurysm, respectively, with A and B being oriented perpendicularly. All the diameters of the aneurysm were measured based on the 3D rotational angiography images.
The volume of the coil was determined using the formula for calculating the volume of a cylinder:
Coil volume = π(diameter/2)2 (length)
The specifications provided by the coil manufacturer were used to obtain the necessary information for this calculation.
Procedure complication encompassed two specific events: [1] extravasation during the procedure and [2] thrombotic or embolic occlusion of distal branches. Temporary reversible vasospasm occurring during the procedure was not included, and no instances of procedure-induced artery dissection were observed.
The RROC was used to evaluate the immediate treatment outcome during the final angiography of the procedure. RROC 1 was indicated complete obliteration of both the sac and neck of the aneurysm. RROC 2 denoted the absence of contrast filling in the aneurysm sac but the presence of contrast filling in the remaining neck. RROC 3 was defined as the presence of contrast filling within the sac [15].
The clinical outcomes were assessed using the mRS at time of discharge, in-hospital mortality, and recanalization during follow-up. Patients were scheduled to undergo angiographic follow-up at 6 and 12 months after discharge, utilizing either CT angiography or MR angiography. All cases followed up for at least 12 months with angiographic imaging. Recanalization was defined as suspected reopening of a sac or an increase in the size of the remaining sac observed during the follow-up CT angiography or MR angiography.
IBM SPSS statistics version 21.0 (IBM, Armonk, NY, USA) was utilized for the statistical analysis. Continuous variables were compared using the student’s t-test for normally distributed valuables, while the Mann-Whitney U test was employed for non-normally distributed values. Categorical variables were compared using the Pearson chi-square test and Fisher’s exact test. Univariate logistic regression analysis was conducted to determine the independent relationship between favorable outcomes and various factors. A p value of <0.05 was considered statistically significant.
Out of the 280 ruptured aneurysms that underwent endovascular procedures, a total of 91 cases met the inclusion criteria for this study. These criteria specified that the aneurysms should be saccular and small, with a diameter of less than 5 mm, and that they should have been treated using either the sMC or the dMC for coil embolization. Among the 91 cases, 67 were treated with sMC and 24 were treated with dMC. Importantly, there were no instances of failed coil embolization in either treatment group. The example cases of each technique are showed at Fig. 1 and Fig. 2.
The demographic characteristics and features of the 91 cases are presented in Table 1. In the sMC group, there were 27 male and 40 female cases, while in the dMC group, there were 11 male and 13 female cases. The mean age of the patients in the sMC group was 54.78 years (median, 59 years; range, 27-87 years), whereas the mean age in the dMC group was 51.5 years (median, 49 years; range, 27-80 years). The average Glasgow Coma Scale (GCS) score at admission was 12.88 for the sMC group and 12 for the dMC group. The mean Hunt and Hess grade and Fisher scale scores at admission were 2.64 and 3.18, respectively, for the sMC group, and 2.71 and 3.29, respectively, for the dMC group.
Among the 91 cases, 9 cases in the sMC group and 5 cases in the dMC group involved aneurysms in the posterior circulation. The mean size of the aneurysms in each group was 3.78 mm and 3.84 mm, with ranges of 2-5 mm and 2.6-4.9 mm, respectively. The mean size of the aneurysmal neck was significantly different between the sMC group (2.168 mm) and the dMC group (2.504 mm) (p <0.05). Additionally, the mean value of the dome-to-neck ratio (D/N ratio) differed between the two groups, with a value of 1.401 in the sMC group and 1.188 in the dMC group (p <0.05).
There were no significant statistical differences observed in the outcome measures between the two groups. The mean coil packing density was higher in dMC group compared to the sMC group (39.94% in sMC and 45.74% in dMC). The mean RROC was 0.52 in the sMC and 0.37 in the dMC, and the distribution of RROC showed differences between the two groups (39 cases with RROC 0, 16 cases with RROC 1, 7 cases with RROC 2 in sMC, 16 cases with RROC 0, 21 cases with RROC 1, and 1 case with RROC 2 in dMC). Procedural complications were more common in the sMC group, with eight cases compared to only one case in the dMC group. The sMC group experienced five cases of extravasation at the ruptured aneurysm and three cases of distal branch occlusion, whereas the dMC group had only one case of extravasation. During the follow-up period, recanalization was observed in seven cases (10.45%) in the sMC and two cases (8.3%) in the dMC. The mean discharge mRS was lower in the dMC group (1.83) compared to the sMC group (1.97), and the mortality rate was higher in the sMC group with four cases (5.97%) compared to one case (4.17%) in the dMC group.
The results of the first subgroup analysis for tiny aneurysms (≤3 mm) are presented in Table 2. Out of the total 91 cases, 27 cases were classified into the tiny aneurysm group. Similar to the overall analysis, the subgroup was divided based on the procedural method. There were no significant differences in demographics between the two groups. However, aneurysmal neck size and the D/N ratio showed statistical differences, consistent with the characteristics of small aneurysms. Additionally, the mean size of the aneurysm differed significantly between the sMC group (2.62 mm) and the dMC group (2.86 mm) (p <0.05). Apart from these findings, no other statistically significant differences were observed in the analyzed features.
The second subgroup analysis was conducted by dividing the dMC group into two subgroups based on whether the aneurysm was tiny (≤3 mm) or not tiny but small (>3 mm, ≤5 mm) Table 3. Since the groups were divided according to size, there was a statistically significant difference in aneurysm size. However, no differences were found in aneurysmal neck size and the D/N ratio between the two subgroups. Coil packing density showed the significant distinction between two groups (56.935% in tiny, 40.14% in not tiny but small aneurysms. No significant differences were observed in the measured outcomes when comparing the aforementioned groups.
In the third subgroup, which analyzed the data further, none of the measured outcomes showed any significant factors in each group, except for coil packing density in the dMC subgroup. Univariate logistic regression analysis was conducted to assess the independent impact of relevant factors on achieving high packing density (coil packing density >40%) Table 4. The only factor found to have a significant effect on high packing density was the size of the aneurysm (odds ratio [OR] 0.309, 95% confidence interval [CI] 0.169-0.566, p <0.05). No statistical significance was observed in the other factors, including the procedural method employed.
The advancements in devices and techniques have contributed to the successful treatment of small aneurysms through endovascular coil embolization [5]. However, there remain several challenges in treating these aneurysms, including their shape, direction, location, and the presence of critical branches on the fundus. In the case of unruptured aneurysms, stent-assisted coil embolization has also made significant progress, providing safer and easier application for a wider range of cases due to advancements in stents and techniques [1,12,14,23]. However, the use of stent-assisted coil embolization necessitates antiplatelet therapy, which can introduce complications or difficulties. Discontinuation of antiplatelet agents may result in thrombotic complications [9]. Moreover, even after successful control of the bleeding focus through coil embolization, the rapid progression of brain swelling and uncontrolled intracranial pressure may require decompressive craniectomy [20]. The use of antiplatelet agents can adversely affect the outcomes of the surgery [21] and potentially have a negative impact on the patient’s prognosis. In cases of ruptured aneurysms, dMC can be considered as an alternative when the conventional sMC encounters challenges in treating small ruptured aneurysms.
The dMC was first described by Baxter et al. in 1998 [2]. Since then, subsequent studies have reported improved outcomes with this technique [7,11,13,24]. One of the key advantages of the dMC is the ability to create a stable framework by interweaving two undetached coils in different directions. This interweaving allows the coils to support each other, resulting in stable packing of frame coils and the ability to safely pack more coils compared to the sMC [7,13]. Another benefit is that the dMC can be performed by simply introducing an additional microcatheter without requiring any special preparation after initial failure with the sMC.
Previous studies have raised concerns about the risk of intraoperative rupture associated with the placement of two microcatheters in small aneurysms [22]. However, in this study, the occurrence of extravasation was only reported in one case (4.17%) in the dMC group and five cases in the sMC group (7.46%). Previous literature reports a possibility of rupture during the procedure to be around 2.5%-3% [7,22]. Although there may be a slightly higher rate in this study, the results suggest that placing two microcatheters into an aneurysmal sac does not significantly increase the risk of rupture. Other complications, such as thrombosis during the procedure or recanalization, were also not frequently observed in the double catheter technique group. Although not statistically significant, there were no significant differences in the average discharge modified Rankin Scale and mortality rates among all groups.
It is worth noting that in this study, all cases in the dMC group were initially attempted with the sMC technique but failed due to an unfavorable configuration. The statistical analysis supported significant differences in the size of the neck and the D/N ratio between the small aneurysm group and the tiny aneurysm group based on the procedural method used. Additionally, the patients in the dMC group had poorer initial clinical status compared to those in the sMC group, as indicated by the GCS scores, Hunt and Hess grades, and Fisher scales. Despite these differences, the outcomes of the dMC group were not inferior to those of the sMC group, which primarily included relatively favorable cases. Furthermore, when comparing outcomes based on size within the dMC group, treating tiny aneurysms with the dMC technique did not result in more hazardous complications or poorer outcomes. One reason for absence of thromboembolic complications in dMC group is that when challenging cases are treated with dMC instead of sMC, it takes less time to insert the same number of coils or to finish the procedure, which is believed to reduce the thromboembolic risk associated with it.
Previous studies have suggested an association between packing density and recanalization [7,19]. However, more recent studies have presented conflicting results [8,10], leading to a controversial understanding of this relationship. Consistent with previous literature, a statistically significant correlation has been observed between smaller aneurysm size and higher packing density [19]. Although not statistically significant, it is expected that the dMC technique allows for the safe insertion of more coils, resulting in higher packing density despite the larger aneurysm size. Long-term follow-up data is needed to assess if these differences have a significant impact, and our center plans to continue monitoring the cases treated with the dMC technique.
This study has several limitations. Firstly, it is a retrospective and observational study, which may limit the ability to establish direct relationships between the factors. Secondly, most outcome measures did not show significant differences, further complicating the interpretation of direct associations. Thirdly, there may be selection bias in the study population regarding aneurysm characteristics, which could introduce potential inaccuracies in the statistical analyses. Additionally, the limited number of cases of tiny ruptured aneurysms in the study makes the analysis less conclusive.
The dMC technique may be valuable for neurointerventionalists, particularly in ruptured aneurysms with unfavorable configurations, as it can be performed without the need for antiplatelet agents. Although this study did not demonstrate superiority of dMC over sMC for small aneurysms, it also did not show inferiority in terms of safety and efficacy. Further multicenter studies focusing on dMC for small ruptured aneurysms are warranted to provide additional insights.
The dMC is a viable option for neurointerventionalists when dealing with aneurysms that have an unfavorable configuration. It provides a safe alternative for cases that are challenging to treat using the sMC, without the need for stent placement or peri-treatment medication in ruptured aneurysm cases. Despite encountering unfavorable configurations and initially poor clinical status in the dMC group, the complications and outcomes were not inferior. Therefore, the dMC can be considered as the primary alternative option for managing difficult small ruptured aneurysms.
Fig. 1.
A simple tiny ruptured aneurysm case treated by sMC. Acom aneurysm was observed in initial angiography. Embolization was done with conventional sMC. sMC, single microcatheter technique; dMC, double microcatheter technique

Fig. 2.
A tiny ruptured aneurysm case treated by dMC. Acom aneurysm was observed in initial angiography. Embolization was done by dMC without any other complication. dMC, double microcatheter technique
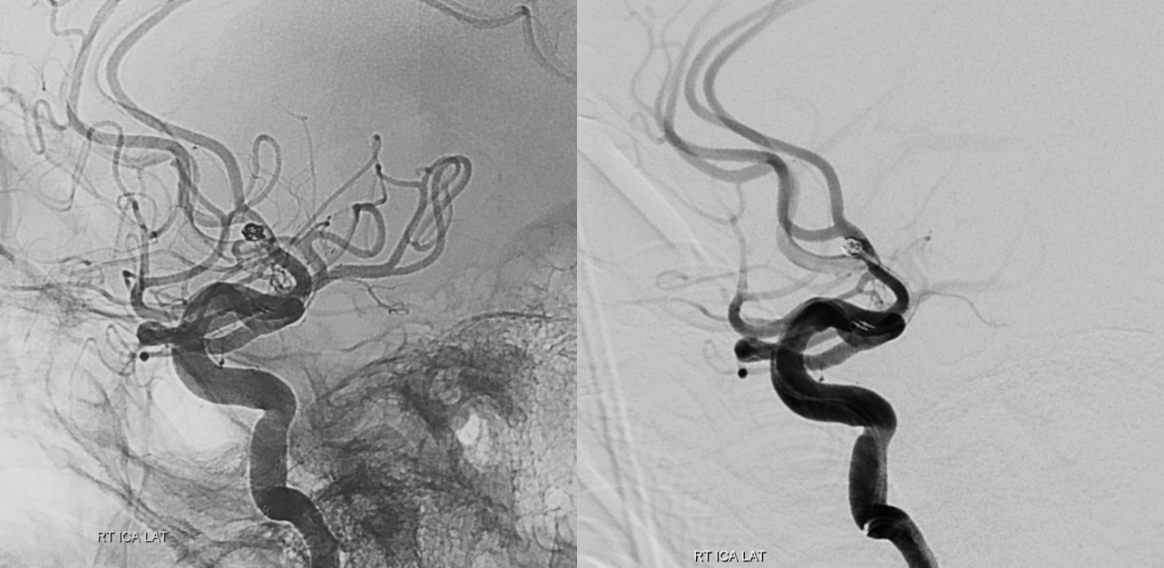
Table 1.
Comparison of sMC and dMC in small ruptured aneurysms (≤5 mm)
Table 2.
Comparison of sMC and dMC in tiny ruptured aneurysms (≤3 mm)
Table 3.
Comparison of tiny ruptured aneurysms (≤3 mm) and not tiny but small ruptured aneurysms (>3 mm, ≤5 mm) in dMC group
Table 4.
Univariate logistic regression analysis for coil packing density in small ruptured aneurysm (≤5 mm) group
REFERENCES
1. Akgul E, Aksungur E, Balli T, Onan B, Yilmaz D, Bicakci S, et al. Y-stent-assisted coil embolization of wide-neck intracranial aneurysms: A single center experience. Interv Neuroradiol. 2011 Mar;17(1):36-48.




2. Baxter BW, Rosso D, Lownie SP. double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. AJNR Am J Neuroradiol. 1998 JunJul;19(6):1176-8.


3. Bechan RS, Sprengers ME, Majoie CB, Peluso JP, Sluzewski M, van Rooij WJ. Stent-assisted coil embolization of intracranial aneurysms: Complications in acutely ruptured versus unruptured aneurysms. AJNR Am J Neuroradiol. 2016 Mar;37(3):502-7.



4. Bodily K, Cloft H, Lanzino G, Fiorella D, White P, Kallmes DF. Stent-assisted coiling in acutely ruptured intracranial aneurysms: A qualitative, systematic review of the literature. AJNR Am J Neuroradiol. 2011 Aug;32(7):1232-6.



5. Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF. Endovascular treatment of very small (3 mm or smaller) Intracranial aneurysms: Report of a consecutive series and a meta-analysis. Stroke. 2010 Jan;41(1):116-21.


6. Chen Z, Feng H, Tang W, Liu Z, Miao H, Zhu G. Endovascular treatment of very small intracranial aneurysms. Surgical Neurology. 2008 Jul;70(1):30-5.


7. Geraghty S, Kreitel KD, Medel R, et al. Single-center experience with a dual microcatheter technique for the endovascular treatment of wide-necked aneurysms. J Neurosurg. 2014 Nov;121(5):1093-101.


8. Goddard JK, Moran CJ, Cross DT 3rd, Derdeyn CP. Absent relationship between the coil-embolization ratio in small aneurysms treated with a single detachable coil and outcomes. AJNR Am J Neuroradiol. 2005 Sep;26(8):1916-20.


9. Golshani K, Ferrel A, Lessne M, Shah P, Chowdhary A, Choulakian A, et al. Stent-assisted coil emboilization of ruptured intracranial aneurysms: A retrospective multicenter review. Surg Neurol Int. 2012 3:84.



10. Hassankhani A, Ghozy S, Bilgin C, Kadirvel R, Kallmes DF. Packing density and the angiographic results of coil embolization of intracranial aneurysms: A systematic review and meta-analysis. Interv Neuroradiol. 2023 Feb;15910199231155288.



11. Kim DJ, Kim BM, Park KY, Ihm EH, Baek JH, et al. Coil embolization of overwide and undertall small intracranial aneurysms with double microcatheter technique. Acta Neurochir (Wien). 2014 May;156(5):839-46.



12. Kühn AL, Hou SY, Puri AS, Silva CF, Gounis MJ, Wakhloo AK. Stent-assisted coil embolization of aneurysms with small parent vessels: safety and efficacy analysis. J Neurointerv Surg. 2016 Jun;8(6):581-5.


13. Kwon OK, Kim SH, Kwon BJ, Kang HS, Kim JH, Oh CW, et al. Endovascular treatment of wide necked aneurysms by using two microcatheters: Techniques and outcomes in 25 patients. AJNR Am J Neuroradiol. 2005 Apr;26(4):894-900.


14. Lee YJ, Kim DJ, Suh SH, Lee SK, Kim J, Kim DI. Stent-assisted coil embolization of intracranial wide-necked aneurysms. Neuroradiology. 2005 Sep;47(9):680-9.



15. Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH, et al. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. 2015 Jul;7(7):496-502.


16. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002 Oct;360(9342):1267-74.


17. Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, et al. Aneurysmal subarachnoid hemorrhage: The last decade. Transl Stroke Res. 2021 Jun;12(3):428-46.



18. Nguyen TN, Raymond J, Guilbert F, Roy D, Bérubé MD, Mahmoud M, et al. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg. 2008 Jun;108(6):1088-92.


19. Rinaldo L, Lanzino G. Increased age associated with reduced likelihood of recurrence after coiling of ruptured aneurysms. World Neurosurg. 2017 Apr;100:381-7.


20. Schirmer CM, Hoit DA, Malek AM. Decompressive hemicraniectomy for the treatment of intractable intracranial hypertension after aneurysmal subarachnoid hemorrhage. Stroke. 2007 Mar;38(3):987-92.


21. Schuss P, Borger V, Vatter H, Singer OC, Seifert V, Güresir E. Antiplatelet therapy, but not intravenous thrombolytic therapy, is associated with postoperative bleeding complications after decompressive craniectomy for stroke. J Neurol. 2013 Aug;260(8):2149-55.



22. Sluzewski M, Bosch JA, van Rooij WJ, Nijssen PCG, Wijnalda D. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: Incidence, outcome, and risk factors. J Neurosurg. 2001 Feb;94(2):238-40.


23. Tähtinen OI, Vanninen RL, Manninen HI, Rautio R, Haapanen A, Niskakangas T, et al. Wide-necked intracranial aneurysms: treatment with stent-assisted coil embolization during acute (<72 hours) subarachnoid hemorrhage—experience in 61 consecutive patients. Radiology. 2009 Oct;253(1):199-208.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 666 View
- 48 Download
- ORCID iDs
-
Hae Won Koo

https://orcid.org/0000-0001-7014-3005 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



