|
 |
| J Cerebrovasc Endovasc Neurosurg > Epub ahead of print |
Abstract
Giant cerebellar cavernomas in children are rare and must be differentiated from hemorrhagic cerebellar tumors. The diagnosis and treatment of giant cerebellar cavernomas is challenging, but complete surgical resection can lead to favorable outcomes and complete neurological recovery in most cases. We present a case of eight months old baby who was diagnosed with a giant cavernoma resulting in secondary obstructive hydrocephalus with neuropsychiatric presentations. The patient underwent a paramedian craniotomy surgery with a suboccipital approach and complete surgical resection of the cavernoma was done. Over nine months of observation, the child showed improvement in their ability to walk and fully recovered from a neurological perspective. We also conducted a literature review to identify eleven cases of giant cerebellar cavernomas in children, including our case. The data were analyzed to determine the clinical features, treatment, and outcomes of giant cerebellar cavernomas in children.
Cavernous malformations of the brain are rare diseases of the central nervous system in children occurring in 0.37-0.9% of the general population according to autopsy and imaging studies [3,6,11,12,15-17,20,21]. In children, they typically range from 9-20 mm in size, with an average diameter of 1.4 cm [11,17,19,23]. Giant cavernous malformations (GCM) larger than 4 cm are rare [2,5,7,18] and we present a case of giant cerebellar cavernoma and review the relevant literature.
The symptoms of GCM in children vary, but may include headache, nausea, vomiting, ataxia, and neck pain. A diagnosis of GCM is typically made through imaging studies, such as magnetic resonance imaging (MRI) and computed tomography (CT) scans. The main treatment for GCM is surgical resection, which is typically performed using a retrosigmoid, median, or paramedian suboccipital approach, depending on the location of the lesion and the experience of the surgeon. The goal of surgical treatment is to achieve complete removal of the cavernoma. Studies have shown that surgical resection of GCM in children can lead to favorable outcomes and complete neurological recovery in most cases. However, there is still much to be learned about the causes, diagnosis, and treatment of GCM, and further research is needed to improve the outcomes for affected children [4,8].
Eight months old baby was diagnosed with a volumetric formation of the posterior cranial fossa, resulting in secondary occlusive hydrocephalus with neuropsychiatric manifestations. The onset of symptoms, including vomiting, irritability, convergent strabismus, increased muscle tone in the legs, and hyperreflexia in the leg tendons and periosteal reflexes, was sudden and reported by the mother to have begun at 2 months of age. An examination of the patient’s fundus showed a pale pink optic disc in both eyes with clear, rounded boundaries and central vessels. The arteries were narrowed while the veins were dilated, tortuous, and showed a ratio of 1:3.5. The patient also had angiopathy of the retinal vessels and venous stasis. The psychologist’s conclusion indicated a violation of psychomotor development of organic origin, characterized by a predominant impairment of motor development with normal speech development.
The patient underwent a MRI of the brain with contrast on the General Electric SIGNA Architect 3T (Boston, Massachusetts, USA) MRI machine. In the projection of the right hemisphere of the cerebellum and its vermis, a cystic-solid formation with multiple cystic inclusions with a horizontal level was detected (Fig. 1).
The patient underwent a paramedian craniotomy surgery in the cervico-occipital region with a suboccipital approach on the right, during which the cavernoma of the right cerebellar hemisphere was removed through microsurgical techniques. During the procedure, a bulging formation was observed on the surface of the brain which appeared dark blue and crimson, while the surrounding surface appeared yellowish. The formation resembled a bunch of grapes and took up almost the entire right hemisphere of the cerebellum. There were cavities filled with lysed blood and hemosiderin. The diagnosis was confirmed by histological examination which revealed a cavernous angioma. The cavities were found to be thin-walled, irregular in shape, and formed by the endothelium. They were tightly adjacent to each other, and some were separated by collagen fibers or fibrous tissue. The cavities were filled with liquid blood and remnants of hematomas from previous hemorrhages were present in the stroma of the formation. Small vessels in the surrounding tissues appeared to be normally formed arterioles and capillaries (Fig. 2). On the 7th day post-surgery, a control MRI of the brain was performed, revealing a bone defect with liquid content in the postoperative bed in the posterolateral parts of the left occipital region, measuring up to 17 mm deep and 62 mm wide. The right hemisphere of the cerebellum had been removed, preserving its pedicle and the stump of the upper part, with cicatricle changes and expansion of the subarachnoid space in this area (Fig. 3).
During the hospital stay, the patient’s symptoms of raised intracranial pressure (eg. vomiting) subsided. On the 18th day, the child was discharged from the hospital after a prolonged stay due to the onset of post-ventilatory bronchopneumonia. Over the course of nine months of observation, the child showed improvement in their ability to walk and fully recovered from a neurological perspective.
Our clinical case and literature review demonstrate that giant cerebellar cavernomas in children require differentiation from tumors with hemorrhage. The size of the giant cavernoma in our case and in Kan et al.’s study [10] was at least 4 cm. The review found that children were affected by the disease between the ages of 1 month and 16 years, with a median age of 6 months. Symptoms of the disease before hospitalization lasted from 1 day to 6 months.
Despite ongoing research in the fields of genetics and embryology, the causes of cavernomas remain unclear. There are two theories regarding the formation of cavernomas. The first theory is that it is a congenital condition, as supported by our case and many other authors, which primarily occur in children under 2 years of age [1,10,13,24]. On the other hand, some researchers believe that cavernomas “grow” into a high-flow malformation (HCM) due to repeated hemorrhages, pseudocapsule formation, and clot expansion driven by an osmotic gradient created by blood breakdown products [9,10,13,22,24]. The occurrence of giant CMs is also well documented in the literature, including in familial cerebral cavernomatosis, which was diagnosed in two cases in our study [14]. This condition is characterized by mutations in the cerebral cavernous malformation (CCM)1/2/3 genes, which increase patients’ risk of developing CMs [14].
Treatment for HCM in our literature review primarily involves surgical resection. Our study demonstrates that resection of giant cerebellar cavernomas can be performed safely using various surgical approaches, such as retrosigmoid, median, or paramedian suboccipital, depending on the location of the lesion and the surgeon’s expertise. The results indicate that the prognosis is generally favorable and complete neurological recovery is achieved in the majority of cases.
In literature review, a search of the PubMed database was conducted to identify studies on giant cavernous angiomas in children. The keywords “giant,” “huge,” “cavernoma,” “cavernous angioma,” and “cerebellar cavernous malformation” were used to identify relevant articles written in English. Studies were included in the review if they reported cavernomas larger than 4 cm in children under 18 years old [19,23]. Eight publications reporting 11 (include our case) cases of giant cerebellar cavernomas in children were identified in the literature review (Table 1). The age of the patients ranged from 1 month to 16 years, with a median age of 7 months and a male-to-female ratio of 1.75:1. The most common symptoms included headache (100%), bulging of the fontanel (45%), macrocephaly (36%), and irritability (27%). Imaging studies showed evidence of hydrocephalus in 55% of the patients. On CT scans, 55% of the cavernomas were hyperdense. On T1W MRI, the lesions appeared as multiple cystic with a heterogeneous structure, and on T2W MRI, the lesions appeared as multicystic with hemosiderin rings surrounded by a hypointense rim. All patients underwent surgical treatment, with 45% receiving ventriculo peritoneal (VPS) or external ventricular drain (EVD) to treat the hypertensive-hydrocephalic syndrome. Total removal of the cavernoma was achieved in 6 patients, with the most common surgical approaches being median suboccipital (4 cases), paramedian suboccipital (3 cases), and retrosigmoid (1 case). The outcome of the treatment was generally favorable, with no fatalities reported at a median follow-up of 12 months. Complete neurological recovery was achieved in 8 cases, and partial recovery was reported in one case.
So from our case we come to conclusion that complete surgical resection of giant cerebellar cavernoma is possible. Surgical resection can also lead to favorable outcomes and complete neurological recovery.
Fig. 1.
MRI of brain T2 FLAIR axial section (A) showed the lesion was 56×54×64 mm.The structure was heterogeneous and the intensity of the area was predominantly heterogeneous. MRI showed signs of a cystic-solid formation with multiple cyst-like inclusions with a horizontal level in the projection of the right hemisphere of the cerebellum and its vermis, ventriculomegaly, and moderate atrophy of the cerebral cortex in both frontotemporal regions. (B) T2 weighted coronal. (C) T1 FLAIR sagittal image showing multiple cystic lesions with fluid level. (D) T1 FLAIR enlargement of lateral and third ventricles with periventricular edema. MRI, magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery
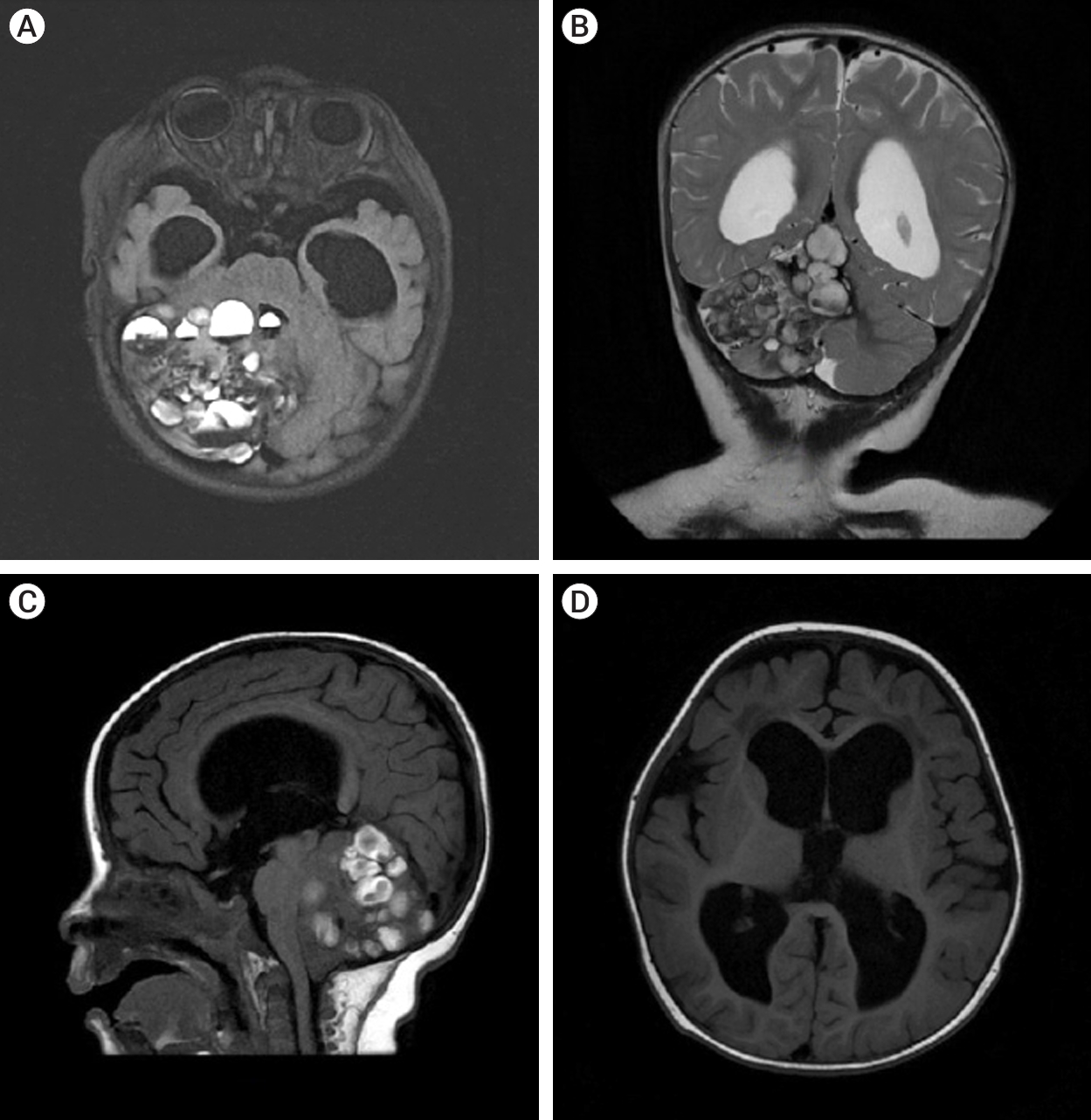
Fig. 2.
Histopathology image: Hematoxylin and eosin staining (10×) demonstrating large, endothelial-lined, capillary-type vessels of various sizes with evidence of erythrocyte breakdown.
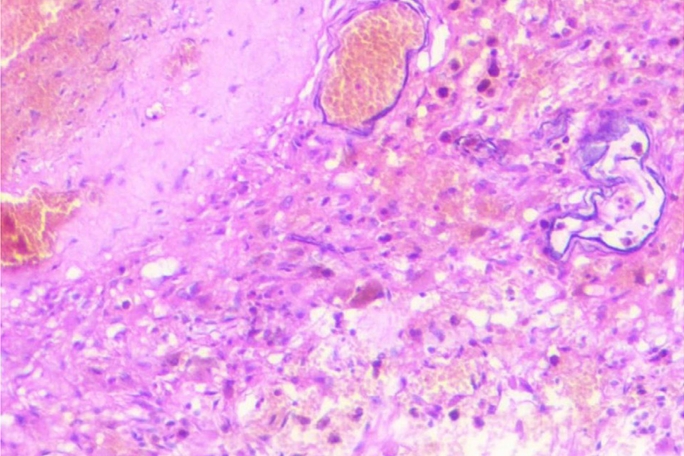
Fig. 3.
Postoperative images showing complete removal of the lesion. (A) T2 axial MRI, (B) T2 FLAIR axial MRI, (C) T2 weighted coronal sections displaying the bed of the removed cavernoma, (D) T2 axial section demonstrating regression of expansion of cerebral ventricles. MRI, magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery
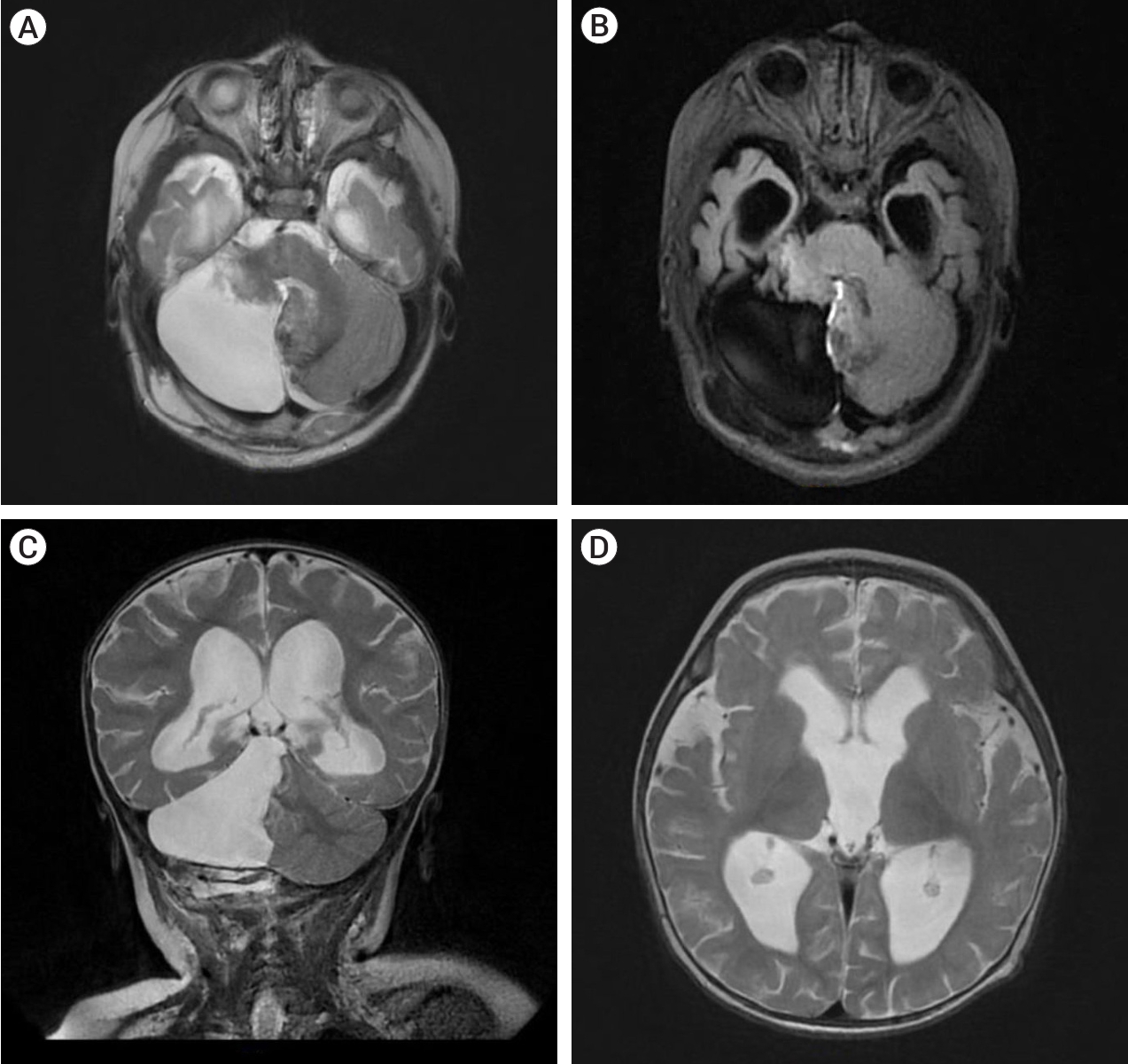
Table 1.
Demographic, clinical, imaging features, treatment, and outcomes of pediatric cerebellar cavernous malformations [4]
| Author/Year | Age/Sex | Country | Clinical features | Duration of symptoms | CT | T1 MRI/T2 MRI | HCP | Largest diameter | Treatment | Outcome | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hayashi et al., 1985 [8] | 6 m/F | Japan | Bulging fontanelle | NR | Hyperdense with homogenous enhancement | N/A | Present | >6 cm | VPS GTE (midline suboccipital) | Partial neurologic recovery | 12 months |
| Macrocephaly | |||||||||||
| Hypotonia Tremor | |||||||||||
| Kan et al., 2008 [10] | 1 m/M | USA | Irritability | NR | Hyperdense, Heterogeneous, Calcification | Hyperintense/Multicystic with multiple hemosiderin rings | NR | 6 cm | Excision | NR | NR |
| Bulging fontanelle | |||||||||||
| Macrocephaly | |||||||||||
| Kan et al., 2008 [10] | 2 m/M | USA | NR | NR | N/A | Hyperintense, Heterogeneous/Multicystic with multiple hemosiderin rings | NR | 4 cm | Excision | NR | NR |
| Atalar et al., 2007 [1] | 6 y/F | Turkey | Headache | 6 months | Hyperdense, Heterogeneous, Calcification | Hypointense irregular enhancement/Multi-cystic with hemosiderin ring | Absent | 6 cm | GTE (midline suboccipital) | Complete neurologic recovery | 12 months |
| Lew, 2010 [14] | 4 m/M | USA | Vomiting Bulging fontanelle | 2 weeks | Hyperdense, Hemorrhagic | Lobulated cystic mass, with subacute hemorrhage/NR | Present | 4 cm | EVD GTE (midline suboccipital) | Complete neurologic recovery | 36 months |
| Opisthotonos | |||||||||||
| Forced gaze deviation | |||||||||||
| Lethargy | |||||||||||
| Lew, 2010 [14] | 7 m/F | USA | Irritability | 1 day | Hyperdense, Hemorrhagic | CT - Hyperdense, Hemorrhagic | Present | >4 cm | EVD STE (midline suboccipital) | Complete neurologic recovery | 16 months |
| Bulging fontanelle | |||||||||||
| Lethargy | MRI - N/A | ||||||||||
| Jurkiewicz et al., 2013 [9] | 4 m/M | Poland | Bulging fontanelle | 1 week | Hyperdense, Hemorrhagic | Hyperintense/Multi-cystic with hypointense rings representing hemosiderin | Present | 6.1 cm | EVD STE (paramedian suboccipital) | Complete neurologic recovery | 2 months |
| Setting sun sign | |||||||||||
| Macrocephaly | |||||||||||
| Villasenor et al., 2017 [24] | 18 m/F | Spain | Cervical torticollis | 6 months | N/A | Multi-cystic with hetereogenous intensity/Multi-cystic with hetereogenous intensity and peripheral hypointense rim | Absent | 5.7 cm | GTE (paramedian, suboccipital) | Complete neurologic recovery | 6 months |
| Upper extremity ataxia | |||||||||||
| Gaddi et al., 2020 [4] | 15 y/M | Philippines | Headache | 3 months | Predominantly isodense with a central hyperdense focus, Minimally contrast enhancing | Multi-cystic with hetereogenous intensity, Predominantly hyperintense/Multi-cystic mixed signal intensities surrounded by a hypointense rim | Present | 5.6 cm | VPS GTE (retrosigmoid, suboccipital) | Complete neurologic recovery | 6 months |
| Dysmetria/ | |||||||||||
| Dysdiadochokinesia | |||||||||||
| Peripheral facial nervepalsy | |||||||||||
| Lethargy | |||||||||||
| Shroff et al., 2021 [22] | 16 y/M | India | Progressive left-sided weakness and left facial palsy | 2 years | N/A | N/A | Absent | 4 cm | Suboccipital | Complete neurologic recovery | 8 years |
| Current study | 8 m/M | Uzbekistan | Vomiting, Irritability, forced position of the head, Macrocephaly | 6 months | N/A | Hyperintense, Heterogeneous/Multicystic with multiple hemosiderin rings | Present | 6.4 cm | GTE (paramedian, suboccipital) | Complete neurologic recovery | 9 months |
REFERENCES
1. Atalar M, Kars Z, Egilmez R, Egilmez H. Giant cavernous angioma mimicking cerebellar neoplasm with major bleed: A case report. Tip Arastirmalari Dergisi. 2007 5(3):153-6.
2. Avci E, Oztürk A, Baba F, Karabağ H, Cakir A. Huge cavernoma with massive intracerebral hemorrhage in a child. Turk Neurosurg. 2007 17(1):23-6.

3. Del Curling O Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991 Nov;75(5):702-8.


4. Gaddi MJS, Pascual JSG, Legaspi EDC, Rivera PP, Omar AT 2nd. Giant cerebellar cavernomas in pediatric patients: Systematic review with illustrative case. J Stroke Cerebrovasc Dis. 2020 Nov;29(11):105264.


5. Gezen F, Karatas A, Is M, Yildirim U, Aytekin H. Giant cavernous haemangioma in an infant. Br J Neurosurg. 2008 Dec;22(6):787-9.


6. Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011 Jun;30(6):e24.


7. Grujić J, Jovanović V, Tasić G, Savić A, Stojiljković A, Matić S, et al. Giant cavernous malformation with unusually aggressive clinical course: A case report. Acta Clin Croat. 2020 Mar;59(1):183-87.


8. Hayashi T, Fukui M, Shyojima K, Utsunomiya H, Kawasaki K. Giant cerebellar hemangioma in an infant. Childs Nerv Syst. 1985 1(4):230-3.


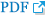
9. Jurkiewicz E, Marcinska B, Malczyk K, Grajkowska W, Daszkiewicz P, Roszkowski M. Giant cerebellar cavernous malformation in 4-month-old boy. Case report and review of the literature. Neurol Neurochir Pol. 2013 Nov-Dec;47(6):596-600.


10. Kan P, Tubay M, Osborn A, Blaser S, Couldwell WT. Radiographic features of tumefactive giant cavernous angiomas. Acta Neurochir (Wien). 2008 Jan;150(1):49-55; discussion 55.


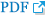
11. Kim DS, Park YG, Choi JU, Chung SS, Lee KC. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997 Jul;48(1):9-17; discussion 17.


12. Konovalov A, Saripov O, Gadzhiagaev V, Titov O, Lasunin N, Zhumabekov A, et al. Optochiasmatic cavernoma: Surgical treatment and outcomes. J Cerebrovasc Endovasc Neurosurg. 2023 Dec;25(4):411-9.



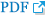
13. Lawton MT, Vates GE, Quinones-Hinojosa A, McDonald WC, Marchuk DA, Young WL. Giant infiltrative cavernous malformation: Clinical presentation, intervention, and genetic analysis: Case report. Neurosurgery. 2004 Oct;55(4):979-80.


14. Lew SM. Giant posterior fossa cavernous malformations in 2 infants with familial cerebral cavernomatosis: The case for early screening. Neurosurg Focus. 2010 Sep;29(3):e18.


15. McCormick WF, Hardman JM, Boulter TR. Vascular malformations (“angiomas”) of the brain, with special reference to those occurring in the posterior fossa. J Neurosurg. 1968 Mar;28(3):241-51.


16. Otten P, Pizzolato GP, Rilliet B, Berney J. [131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies]. Neurochirurgie. 1989 35(2):82-3, 128-31. Article in French.

17. Ozgen B, Senocak E, Oguz KK, Soylemezoglu F, Akalan N. Radiological features of childhood giant cavernous malformations. Neuroradiology. 2011 53(4):283-9.


18. Ozsoy KM, Oktay K, Gezercan Y, Cetinalp NE, Olguner SK, Erman T. Giant cavernous malformations in childhood: A case report and review of the literature. Pediatr Neurosurg. 2017 52(1):30-5.


19. Parizel MR, Menovsky T, Van Marck V, Lammens M, Parizel PM. Giant cavernous malformations in young adults: Report of two cases, radiological findings and surgical consequences. JBR-BTR. 2014 Sep-Oct;97(5):274-8.


20. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: Natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997 Aug;87(2):190-7.


21. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991 Nov;75(5):709-14.


22. Shroff K, Deopujari C, Karmarkar V, Mohanty C. Paediatric giant cavernomas: Report of three cases with a review of the literature. Childs Nerv Syst. 2021 Dec;37(12):3835-45.


-
METRICS

-
- 2 Crossref
- 0 Scopus
- 554 View
- 19 Download
- ORCID iDs
-
Bipin Chaurasia

https://orcid.org/0000-0002-8392-2072 - Related articles
-
Choroid plexus arteriovenous malformations: A systematic review2023 December;25(4)
Sinus Pericranii: A Case Report and the Literature Review.2009 December;11(4)
Cavernous Sinus Thrombophlebitis: Case Report and Literature Review.2010 September;12(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print