 |
 |
| J Cerebrovasc Endovasc Neurosurg > Epub ahead of print |
Abstract
Objective
We evaluated the role of subgaleal closed suction drains in postoperative epidural hematoma (EDH) and wound complications following pterional craniotomy for cerebral aneurysm.
Methods
We reviewed 5,280 pterional craniotomies performed on 5,139 patients between January 2006 and December 2020. A drain was placed subgalealy and tip of drain was positioned between the bone flap and the deep temporalis. 1,637 cases (31%) had a subgaleal suction drain. We analyzed demographic and clinical variables related to EDH requiring evacuation and wound complications in patients with and without drains. Univariate and multivariate logistic regression analyses were performed to determine the associated risk factors.
Results
Fourteen cases (0.27%) of EDH requiring evacuation and 30 cases (0.57%) of wound complications were identified. Univariate analysis found that drain insertion, subarachnoid hemorrhage (SAH), and operation time were associated with EDH, while drain insertion, SAH, male gender, older age, and longer operation time were associated with wound complications. Multivariate analysis found no significant association between drain use and EDH (OR=1.62, p=0.402) or wound complications (OR=1.45, p=0.342).
The pterional craniotomy, popularized by Yasargil in the mid-1970s, is a common approach for treating cerebral aneurysms due to its simplicity and effectiveness [12,13]. However, it carries risks of hematomas from the sphenoid ridge bleeding and bleeding from the detached temporalis muscle, as well as complications from fluid accumulation in the surgical wound. Many neurosurgeons insert surgical site suction drains to mitigate these complications. Despite their widespread use in various surgeries for preventing fluid accumulation and aiding wound healing, their efficacy following pterional craniotomy is underexplored.
Although subgaleal drains have been used in clinical practice, there is a lack of consensus regarding their use, and their potential benefits and drawbacks remain uncertain. While surgical site suction drains have been found to prevent the occurrence of fluid accumulation or bleeding that causes complications [1,8], they are also considered foreign bodies that may promote infection [11]. Therefore, this study aims to investigate the effect of subgaleal suction drains on the occurrence of epidural hematoma (EDH) requiring evacuation and wound complications in patients undergoing pterional craniotomy for the treatment of cerebral aneurysm. Through retrospective analysis, we aim to clarify the optimal use of these drains in this surgical context.
This retrospective study was approved by the Institutional Review Board of our center (IRB No. 2021-0663). The study included patients who underwent pterional craniotomy for the treatment of cerebral aneurysms between January 2006 and December 2020 at our center. Patients who underwent a surgical approach other than pterional craniotomy, expired within 14 days after surgery, underwent postoperative craniotomy extension due to brain swelling, had underlying coagulopathy, or underwent extracranial-intracranial or intracranial-intracranial bypass were excluded from the study.
A subgaleal suction drain was utilized in all surgeries performed before May 2013, whereas its use was discontinued after this time period. Since May 2013, a subgaleal suction drain was selectively applied based on the surgeonŌĆÖs discretion regarding whether antiplatelet drugs or anticoagulants were being taken, or if there was a tendency to bleed.
Demographic characteristics were collected retrospectively from electronic medical records, including information on sex, age, weight, height, and body mass index (BMI). The presence of underlying diseases, such as diabetes mellitus (DM), hypertension, antiplatelet or anticoagulation agent intake, immunosuppressant use, smoking, and time of operation were documented as variables to identify any significant confounding variables that affect EDH requiring evacuation and wound complications. Computed tomography (CT) of the brain was routinely performed immediately after surgery and on postoperative day 3. Supplementary brain imaging was conducted as required based on clinical indications, such as the presence of severe headache, neurological decline, or indications suggesting a potential surgical site infection.
Patients were instructed to stop taking antiplatelet agents for at least 7 days and anticoagulants (including warfarin and direct oral anticoagulants) for at least 48 hours before elective surgeries for non-emergent unruptured aneurysms. Platelet transfusions were done during emergency surgeries for individuals who were receiving antiplatelet medications. Antidotes and fresh frozen plasma were administered to patients who were using anticoagulants in order to regulate the prothrombin time international normalized ratio at a normal level.
The craniotomies were conducted in the operating room under controlled laminar air flow conditions. To reduce the risk of deep vein thrombosis, an intermittent pneumatic compression device was used for all patients, and low molecular weight heparin was not administered. Prophylactic antibiotics were given within one hour before making the incision on the scalp. Cefazolin, a first-generation cephalosporin, was injected repeatedly every four hours. Ciprofloxacin was injected every eight hours in patients with cephalosporin allergy.
If a patient had a drain, antibiotics followed a set schedule until the drainŌĆÖs removal, as per local protocol. However, antibiotics were discontinued within 24 hours after surgery in patients without drain insertion. Prior to elective surgeries, patients performed a hair and scalp cleansing using betadine soap on the day preceding the operation. Hair and scalp washing was not allowed prior to emergent surgeries, or to patients with betadine allergy. To prepare the scalp incision based on the surgeonŌĆÖs preference, the scalp was shaved in either of the following methods: complete shaving of the frontotemporal area or partial shaving with a width of 1-2 cm along the designated incision line. After the shaving process, the incision site was cleansed using a solution containing alcohol and iodine derivatives. Subsequently, an iodine-impregnated incision drape called Ioban┬« (3-M, St. Paul, MN, USA) was applied to cover the prepared area. After the intradural procedure was completed, the intradural space was filled with warm saline. The dura was closed watertightly, using a continuous suture of Prolene 4-0. Subsequently, the bone flap was secured with a screw and plate system. In cases that required drainage, the Jackson-Pratt drain was positioned subgaleally, filling the space between the bone flap and the underlying temporalis muscle fascia. The drain tubeŌĆÖs end was situated near the drilled portion of the lateral sphenoid wing, serving to facilitate communication with the epidural space. The remote end of the drain tube was linked to the trocar, then passed through the scalp and connected to a closed 200 mL bulb suction system. Temporalis muscle was directly reattached with Vicryl 2-0 sutures. Interrupted sutures made with 3-0 Vicryl were used for both the subgaleal and subcutaneous layers. The skin was then closed with 3-0 Nylon sutures or, in some cases, with staples. Compressive dressings were applied in most of cases. Saline irrigation of surgical field was frequently performed during surgery in all patients, particularly after dura closure, bone flap closure, and temporalis muscle suture.
The operated area was inspected on a daily basis, and the wound dressings were regularly replaced. The compression bandages were taken off a day following the surgical procedure. The amount of drained fluid was measured every 8 hours and the reservoir was emptied for the drain group. If the drainage volume was less than 100 mL in the prior 24 hours and below 30 mL in the preceding 8 hours, the drains were taken out in a sterile environment. All drains were withdrawn at least after 48 hours, and the exit site was closed with a single suture. The sutures or staples placed on the scalp were typically taken out between the seventh and tenth days following the operation.
Two outcome variables were recorded: first, the occurrence of EDH requiring evacuation; and second, the occurrence of wound complications. Complications related to the wound were categorized as any of the subsequent conditions: a surgical site infection, which is evidenced by positive cultures from surgical samples, pus or clear liquid discharge from the wound accompanied by local heat, redness, sensitivity, or subdural empyema, and wound dehiscence, characterized by either spontaneous or medically induced separation of the skin layer that necessitates re-closure. Revisional surgery was carried out to manage epidural fluid accumulation or hematoma in individuals experiencing severe headaches or focal neurologic dysfunction due to the mass effects.
All statistical analyses were performed using R Version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). Independent-sample t-test was used for the baseline characteristics; Wilcoxon rank sum test was used for continuous variables, and the chi-squared test or FisherŌĆÖs exact test was used for categorical variables. Univariate logistic regression analyses were conducted to identify the risk factors associated with the two outcome variables. The factors analyzed included the drain insertion, sex, age, BMI, the presence of hypertension, DM, smoking, use of antithrombotic agent, use of immunosuppressant, craniotomy side, and the duration of operation. If the univariate analyses identified factors other than drain insertion was associated with the two outcome variables with p-value <0.10, multivariate logistic analyses were performed further to identify the association with drain insertion and the two outcome variables adjusted for those variables. A p-value <0.05 was considered statistically significant.
A total of 5,280 pterional craniotomies were performed in 5,139 patients for clipping of cerebral aneurysms. Of these, 1,637 cases (31%) were assigned to the subgaleal suction drain (SGSD) group and 3,643 cases (69%) were assigned to the no drain (ND) group.
Baseline characteristics of the SGSD group and the ND group were compared. The prevalence of subarachnoid hemorrhage (SAH) was significantly higher in the SGSD group compared to the ND group (19% vs 5.5%, p<0.001). The mean age was significantly lower in the SGSD group compared to the ND group (55.9┬▒10.0 years vs 58.6┬▒9.57 years, p<0.001), while the mean BMI was significantly lower in the SGSD group compared to the ND group (24.5┬▒3.2 kg/m2 vs 24.8┬▒3.3 kg/m2, p=0.003). The prevalence of hypertension was significantly lower in the SGSD group compared to the ND group (48.1% vs 53.8%, p<0.001). The sex, prevalence of diabetes, smoking and the proportion of patients who received immunosuppressant agents were similar in both groups. The proportion of patients who received antiplatelet agents was similar in both groups (p=0.154), while the proportion of patients who received anticoagulant agents was significantly lower in the SGSD group compared to the ND group (p=0.005). The mean time of operation was significantly longer in the SGSD group compared to the ND group (244.4┬▒73.3 vs 219.9┬▒60.4 minutes, p<0.001). There was a single case with catheter-related complication, which was while the drain tube was being removed, it was caught on the edge of the bone flap, leaving a part of the distal end, which was removed through revisional surgery. The baseline characteristics between SGSD and ND group are detailed in Table 1.
Among all patients, 14 patients (0.27%) had surgical complication of EDH requiring evacuation. Eight cases (0.5% of all SGSD cases) occurred in the SGSD group and 6 cases (0.2% of all ND cases) occurred in the ND group. Fig. 1 shows the brain CT of the representative patient.
The univariate analysis showed that the presence of SAH (OR=12.57, 95% CI=4.36-38.31, p<0.001), drain insertion (OR=2.98, 95% CI=1.03-9.06, p=0.044) and time of operation (OR=1.01, 95% CI=1.00-1.01, p<0.001) were significantly associated with EDH requiring evacuation. In the multivariate analysis, the presence of SAH (OR=10.72, 95% CI=3.50-34.58, p<0.001) the time of operation (OR=1.01, 95% CI=1.00-1.01, p<0.001) was significantly associated with EDH requiring evacuation. The association between drain insertion and EDH requiring evacuation was no longer significant in the multivariate analysis (OR=1.62, 95% CI=0.53-5.21, p=0.402) (Table 2).
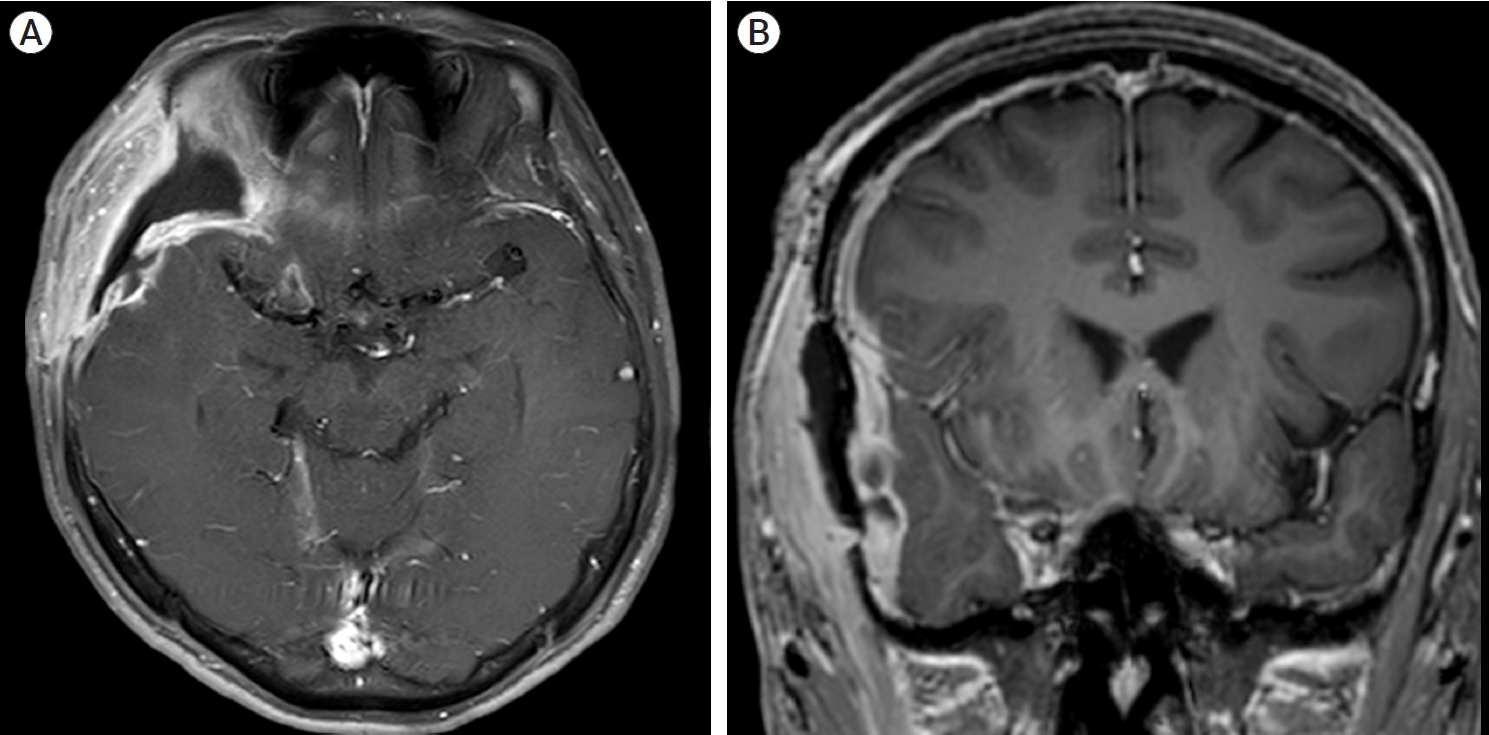
A total of 30 (0.57%) patients had wound complications among the 5,280 craniotomies. Fifteen cases (0.9% of all SGSD cases) occurred in the SGSD group and 15 cases (0.4% of all ND cases) occurred in the ND group. Fig. 2 indicates the brain magnetic resonance imaging (MRI) of the representative case of surgical site infections (SSIs).
The univariate analysis showed that drain insertion (OR=2.24, 95% CI=1.08-4.62, p=0.028), the presence of SAH (OR=7.27, 95% CI=3.44-14.99, p<0.001), male sex (OR=2.33, 95% CI=1.13-4.82, p=0.021), age (OR=0.96, 95% CI=0.93-0.99, p=0.021) and time of operation (OR=1.004, 95% CI=1.00-1.01, p=0.033) were significantly associated with wound complications. In the multivariate analysis, the presence of SAH (OR=6.12, 95% CI=2.79-13.18, p<0.001), male sex (OR=2.12, 95% CI=1.02-4.40, p=0.042), were found to be significant risk factors for wound complications. The association between drain insertion and wound complications was no longer significant in the multivariate analysis (OR=1.45, 95% CI=0.67-3.11, p=0.342) (Table 3).
A subgroup analysis was performed on cases with SAH. Out of the total 5,280 cases, 513 (9.72%) were aneurysmal SAH cases, and 311 SAH cases (60.6% of total SAH cases) had a subgaleal suction drain. The EDH requiring evacuation event occurred in 8 cases (1.56% of total SAH cases), and wound complications occurred in 13 cases (2.53% of total SAH cases). There were no significant differences in baseline characteristics between the two groups (Table 4). Univariate analysis showed that the use of a subgaleal suction drain was not associated with EDH requiring evacuation (OR=4.63, 95% CI=0.82-86.85, p=0.153) or wound complications (OR=3.67, 95% CI=0.97-23.85, p=0.093) (Table 5).
In this retrospective study, we aimed to investigate the effect of subgaleal suction drains on the occurrence of EDH requiring evacuation and wound complications in patients undergoing pterional craniotomy for the treatment of cerebral aneurysm. We hypothesized that subgaleal suction drain would reduce postoperative hemorrhage and fluid collection, thereby preventing complications such as EDH and wound complications. Our results suggest that the use of subgaleal drains is not associated with a significant reduction in the occurrence of EDH requiring evacuation or wound complications.
Postoperative EDH is a rare but serious complication of pterional craniotomy, which may result in neurological deficits or death if left untreated. The presence of SAH and longer time of operation were identified as significant risk factors for EDH requiring evacuation, while the use of subgaleal drain was not associated with a significant reduction in the occurrence of this complication. This finding is in line with a previous study by Choi et al., which reported that the use of subgaleal drain did not reduce the incidence of EDH requiring evacuation in patients undergoing pterional craniotomy for the treatment of ruptured cerebral aneurysms [3]. However, our study is meaningful by adding evidence on elective unruptured cerebral aneurysms cases to existing studies. And this is the largest volume those studied so far.
Presence of SAH was an important risk factor, the postoperative hemorrhagic risk was thought to be high because antiplatelet or anticoagulation agent could not be properly stopped before surgery, unlike elective surgical cases [4,9]. In addition, the possibility of an increase in postoperative hemorrhagic complications due to imbalance of coagulation and fibrinolysis systems could be considered [6]. Also, this could be a possible cause because SAH cases itself usually takes longer operation time. As the operation time increases, more cerebrospinal fluid (CSF) loss is inevitable during surgery, resulting in the space between dura and bone to be more vulnerable to detachment. This detachment and low pressure in the epidural space caused by CSF loss are thought to affect the development of EDH. Longer operation time is also associated with more blood loss in surgical field. Patients might have received substantial colloid administration or blood transfusion, which could disrupt the normal clotting process, consequently increasing the risk of an EDH.
Wound complications are another common complication of pterional craniotomy, which may prolong hospital stay, increase healthcare costs, and impair patient outcomes. In our study, the use of subgaleal suction drain was not associated with a significant reduction in the occurrence of this complication. This finding is also consistent with a previous study by Kim et al., which reported that the use of subgaleal drain did not reduce the incidence of wound complications in patients undergoing pterional craniotomy for the treatment of ruptured cerebral aneurysms [3]. Another study in patients undergoing supratentorial craniotomy reported that the use of subgaleal drain was not associated with a promotion of wound healing or a reduction in wound complications [5].
The presence of SAH and male sex were identified as significant risk factors for wound complications. It might be cause of preoperative bathing using antimicrobial soap the day before surgery in elective surgery cases; however, it was not possible in ruptured cases [10]. The whole hair removal was performed in a ruptured case, and it was thought that microorganism entered and colonized through microtrauma of the scalp occurred during hair removal, causing surgical site infection [2]. One possible explanation for this gender difference is that male patients may have more abundant hair growth in the scalp region, which can increase the risk of SSIs. According to a study published in the Journal of British Neurosurgery, male patients had a significantly higher rate of SSIs following craniotomy [7].
This study possesses numerous merits. Firstly, it encompasses the most extensive pool of patients with either ruptured or unruptured cerebral aneurysms who underwent clipping through pterional craniotomy. Secondly, the surgeries were performed at one single location by three skilled neurovascular specialists, ensuring a uniform technical approach to the procedures. Thirdly, policies pertaining to perioperative management (like discontinuation of antiplatelet or anticoagulant agents, hemostasis, blood pressure regulation, and wound dressing techniques) remained consistent throughout the duration of the study. To minimize potential bias, all electronic medical records and data were evaluated by an individual not affiliated with the center during the studyŌĆÖs duration. However, the study also has its limitations. Firstly, this study is based on a retrospective design, inherently associated with potential bias derived from its structure. Secondly, the absence of subgaleal drain insertion in the studyŌĆÖs later stages could lead to potential bias related to the timing of the procedure. Thirdly, patient randomization was not feasible. As a result, a comprehensive, meticulously planned, multi-center, and prospective randomized controlled trial is warranted to determine the risks and advantages of using a subgaleal drain in patients undergoing pterional craniotomy.
Fig.┬Ā1.
Brain computed tomography (CT) of a patient with postoperative epidural hematoma (EDH) requiring evacuation.
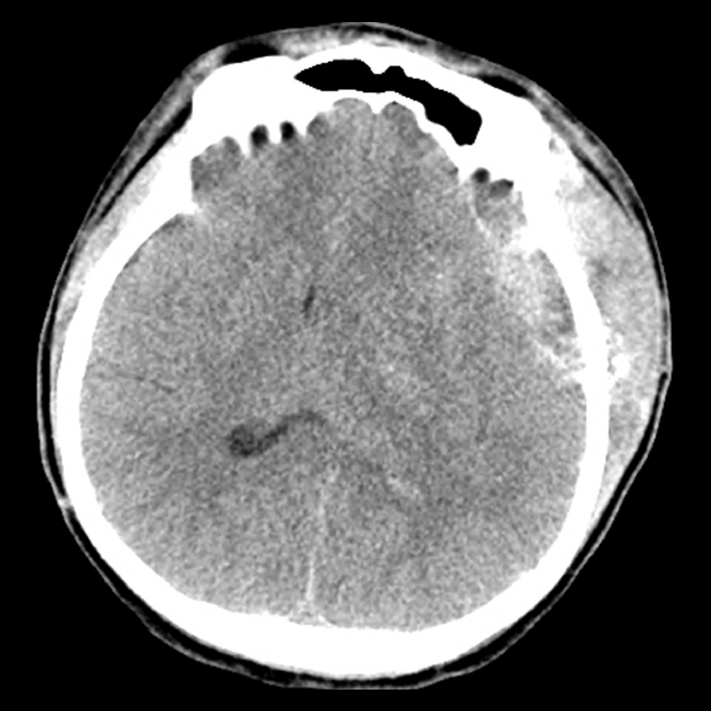
Fig.┬Ā2.
Magnetic resonance imaging (MRI) of a patient with postoperative epidural abscess, (A) T1-enhanced axial image, (B) T1-enhanced coronal image. Approximately 6 cm-extent fluid collection with irregular thickened peripheral enhancement is observed around the operative site.
Table┬Ā1.
Baseline characteristics of subgaleal suction drain group and no drain group
Table┬Ā2.
Logistic regression analysis of factors influencing the occurrence of epidural hematoma (EDH) requiring evacuation
Table┬Ā3.
Logistic regression analysis of factors influencing the occurrence of wound complications
Table┬Ā4.
Baseline characteristics of subgaleal suction drain group and no drain group in SAH cases
Table┬Ā5.
Univariate analysis of factors influencing the occurrence of EDH requiring evacuation and wound complications in SAH cases
REFERENCES
1. Alexander JW, Korelitz J, Alexander NS. Prevention of wound infections. A case for closed suction drainage to remove wound fluids deficient in opsonic proteins. Am J Surg. 1976 Jul;132(1):59-63.

3. Choi SY, Yoon SM, Yoo CJ, Park CW, Kim YB, Kim WK. Necessity of surgical site closed suction drain for pterional craniotomy. J Cerebrovasc Endovasc Neurosurg. 2015 Sep;17(3):194-202.



4. Gerlach R, Scheuer T, Beck J, Woszczyk A, Seifert V, Raabe A. Risk of postoperative hemorrhage after intracranial surgery after early nadroparin administration: Results of a prospective study. Neurosurgery. 2003 Nov;53(5):1028-34; discussion 103-4.



5. Hamou HA, Kotliar K, Tan SK, Wei├¤ C, Christian B, Clusmann H, et al. Surgical nuances and placement of subgaleal drains for supratentorial procedures-a prospective analysis of efficacy and outcome in 150 craniotomies. Acta Neurochir (Wien). 2020 Apr;162(4):729-36.




6. Ji Y, Meng QH, Wang ZG. Changes in the coagulation and fibrinolytic system of patients with subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2014 Jun;54(6):457-64.



7. Korinek AM, Golmard JL, Elcheick A, Bismuth R, van Effenterre R, Coriat P, et al. Risk factors for neurosurgical site infections after craniotomy: A critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg. 2005 Apr;19(2):155-62.


8. Norman G, Goh EL, Dumville JC, Shi C, Liu Z, Chiverton L, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2020 Jun;6(6):CD009261.



9. Palmer JD, Sparrow OC, Iannotti F. Postoperative hematoma: A 5-year survey and identification of avoidable risk factors. Neurosurgery. 1994 Dec;35(6):1061-4; discussion 106-4.

10. Schaffzin J, Simon K, Conelly B, Mangano F. Standardizing preoperative preparation to reduce surgical site infections among pediatric neurosurgical patients. J Neurosurg Pediatr. 2017 Apr;19(4):399-406.


11. Schipmann S, Akalin E, Doods J, Ewelt C, Stummer W, Suero Molina E. When the infection hits the wound: matched case-control study in a neurosurgical patient collective including systematic literature review and risk factors analysis. World Neurosurg. 2016 Nov;95:178-89.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 316 View
- 9 Download
- ORCID iDs
-
Wonhyoung Park

https://orcid.org/0000-0002-9977-0595 - Related articles
-
Necessity of Surgical Site Closed Suction Drain for Pterional Craniotomy2015 September;17(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



