 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(4); 2023 > Article |
|
Abstract
Objective
This study aimed to develop microsurgical strategies based on the anatomical relationship between dorsal internal carotid artery (ICA) aneurysms, the falciform ligament (FL), and the anterior clinoid process (ACP).
Methods
Between 2017 and 2022, 25 patients with unruptured dorsal ICA aneurysms (less than 4 mm in diameter) underwent microsurgical direct clipping. These cases involved the left ICA (n=17) and the right ICA (n=8), with a mean aneurysm size of 3.3 mm (range, 2.5 to 4 mm). We used computed tomography angiography (CTA) and digital subtraction angiography to elucidate the anatomical relationship between dorsal ICA aneurysms and other structures. All procedures involved an ipsilateral pterional approach with securement of the ipsilateral cervical ICA for proximal control.
Results
Among the 25 dorsal ICA aneurysms, 8 (32%) were clipped without the FL being incised. Another 5 (20%) were clipped solely after the FL was cut. For the remaining 12 cases, the aneurysms were successfully clipped following FL incision and partial ACP removal. Patients exhibited favorable postoperative recoveries with good outcomes, and postoperative CTA revealed complete aneurysm clipping without any residual remnants.
Conclusions
We were able to perform clipping without removing the ACP in 13 patients (52%), and in 8 of these (32%), the clipping was carried out directly without cutting the FL. Microsurgery, coupled with proximal control of the cervical ICA, can serve as a viable alternative for patients with small dorsal ICA aneurysms, especially when endovascular treatment options are limited, and 3D CTA confirms a clear anatomical relationship with the ACP.
Despite the recent inclination towards endovascular treatment for paraclinoid aneurysms, microsurgical treatment may still be necessary in certain instances [3,4,12,14]. Long-term antiplatelet therapy is required for cases wherein stent-assisted coiling is performed, and these cases also pose a risk of recurrence [13]. As such, clipping is a slightly better choice for younger patients or those with challenging coil placement due to tortuous vessels. Specifically, for aneurysms with diameters of 4 mm or less, the risk of intraoperative rupture during coil placement increases, making clipping preferable [11]. The surgical difficulty of clipping dorsal internal carotid artery (ICA) aneurysms varies based on whether the anterior clinoid process (ACP) is removed. Barring the challenge of ACP removal, the use of a dorsal ICA aneurysm clip provides an important advantage as it enables quicker aneurysm exposure without the need for Sylvian fissure dissection, given that the carotid and optic cistern can be readily accessed.
This study aimed to investigate the surgical outcomes of microsurgery performed on unruptured dorsal ICA aneurysms with diameters of 4 mm or less, to help establish surgical strategies based on the anatomical relationship between the aneurysm and the ACP.
This study, approved by the relevant institutional review board and exempted from requiring informed consent due to its retrospective design, was a retrospective analysis of 25 patients with unruptured dorsal ICA aneurysms that were 4 mm or less in diameter. These patients were selected from a group of 42 consecutive patients with superior paraclinoid aneurysms who underwent microsurgery between 2017 and 2022. The mean patient age was 54.4 years (range, 34 to 76 years), with a gender distribution of 9 men and 16 women. There were 17 cases involving the left ICA and 8 cases involving the right ICA. The mean aneurysm size was 3.3 mm (range, 2.5 to 4 mm). Before surgery, all patients underwent computed tomography angiography (CTA) to assess the relationship between the aneurysm, ophthalmic artery, and ACP. Three-dimensional (3D) CTA with bone images proved highly valuable in devising surgical strategies, as it provided a clear depiction of the anatomical relationships between the aneurysm, ICA, and ACP. Digital subtraction angiography (DSA) was performed on 11 patients, and 3D DSA images aided in allowing for more accurate determination of the projection type of aneurysm by confirming its proximity to the ophthalmic artery.
Preoperatively and postoperatively, all patients were subjected to neurological examinations to monitor changes in consciousness and potential optic nerve damage. To confirm the success of the clipping, postoperative CTA was performed for all patients.
A pterional approach on the ipsilateral side was employed for all patients. To ensure proximal control without the need for additional ACP removal, the cervical ICA was meticulously exposed and secured from the ipsilateral neck. Following dural opening, the Sylvian fissure was left intact, and the carotid and optic cisterns were directly opened to expose the optic nerve and ICA. If immediate exposure of the aneurysm allowed possible neck clipping, direct neck clipping was performed without removing the falciform ligament or ACP after a temporary clip was placed on the cervical ICA (Fig. 1A, B). If the aneurysm was partially obscured by the falciform ligament, the ligament was incised using a sickle knife to expose the aneurysm. If this exposure was adequate for direct clipping without further ACP removal, a temporary clip was placed on the cervical ICA, and direct clipping was performed (Fig. 1C, D, E). If full exposure of the aneurysm remained insufficient even after cutting the falciform ligament, partial ACP drilling was conducted, followed by the same clipping method (Fig. 1F, G, H, I). Care was taken during aneurysm clipping to avoid optic nerve damage. Given the tendency of dorsal ICA aneurysms to occur at branch-free locations, the clips were positioned as parallel as possible to the ICA to prevent clip slippage and ICA twisting after the aneurysm was covered with a thin slice of cotton.
Among the 25 dorsal ICA aneurysms treated with microsurgical clipping, 8 (32%) were immediately exposed upon opening the carotid and optic cistern, allowing for clipping without the need to remove the falciform ligament or ACP. Clipping was possible after solely cutting the falciform ligament in 5 cases (20%), while in the remaining 12 cases (48%), partial ACP removal was performed after cutting the falciform ligament due to incomplete aneurysm exposure (Fig. 2). ACP removal was deemed unnecessary if the aneurysm was located distal to the ACP in all patients’ 3D CTA with bone images. Clipping was successfully performed for all patients, and postoperative CTA confirmed the complete clipping of the aneurysms without remnants. Postoperative assessments revealed modified Rankin Scale scores of 0 for all patients, with no significant postoperative complications, such as cerebral infarction, postoperative hemorrhage, or visual symptoms.
For each patient, the following data are summarized in Table 1: age, sex, aneurysm location, size, ACP removal status, falciform ligament cutting status, complications, postoperative modified Rankin Scale score, and visual symptoms.
The patient, a 38-year-old woman, had a 2.5-mm aneurysm on the right ICA, detected by brain magnetic resonance angiography (MRA) during a routine health checkup. Preoperative DSA revealed a dorsal ICA aneurysm distal to the ophthalmic artery (Fig. 3A). As per 3D CTA with bone image this aneurysm was located distal to the ACP, suggesting that ACP removal would not be necessary during surgery (Fig. 3B). We used the right pterional approach. The aneurysm was immediately exposed after opening the carotid and optic cisterns (Fig. 3C). We first wrapped the aneurysm in a thin slice of sheet to prevent clip slippage, then clipped it completely without cutting the falciform ligament or removing the ACP (Fig. 3D). The patient had an uneventful postoperative course and was discharged without any neurological deficits.
A 54-year-old woman presented with a 3-mm unruptured dorsal ICA aneurysm identified on CTA. This aneurysm was located distal to the ACP on 3D CTA with bone image, suggesting that ACP removal would not be necessary (Fig. 4A). In the operative field, the aneurysm was not fully exposed due to its proximity to the falciform ligament (Fig. 4B). After the falciform ligament was cut, the aneurysm was fully exposed without ACP removal and successfully clipped (Fig. 4C, D). The patient recovered well and was discharged without any neurological deficits.
A 34-year-old woman presented with a 4-mm unruptured dorsal ICA aneurysm detected on brain MRA. Preoperative DSA revealed the aneurysm with a bleb located distal to the ophthalmic artery (Fig. 5A). 3D CTA with bone image revealed that the aneurysm was medial, not distal, to the ACP, suggesting that partial removal of the ACP would be required (Fig. 5B). As anticipated, the aneurysm was observed intraoperatively to be partially obscured by the falciform ligament and ACP (Fig. 5C). Initially, the falciform ligament was cut with a sickle knife. As the aneurysm was still not fully exposed, the ACP was partially removed using a highspeed drill, which sufficiently exposed the aneurysm (Fig. 5D). Finally, the aneurysm was completely clipped without any difficulty (Fig. 5E). The patient had an uneventful postoperative course and was discharged without any neurological deficits.
According to Krisht’s classification, superior paraclinoid aneurysms of the ICA include both ophthalmic and dorsal ICA aneurysms [5]. Recently, endovascular treatment has been regarded as the primary treatment for these aneurysms [10,16]. The advent of stent-assisted coil embolization and flow diverters has facilitated treatment [9,15]. However, coil embolization of dorsal ICA aneurysms with diameters less than 4 mm presents technical difficulties [11]. In many cases, shaping the microcatheter is necessary, and stent assistance is often required [1,7]. While flow diverters are a good alternative, young patients may need to take antiplatelet agents for an extended period, and periodic examinations may be necessary to monitor for recurrence [13]. In the surgical findings of most patients with dorsal ICA aneurysms, optic nerve compression by the aneurysm is present without preoperative visual symptoms. Therefore, for relatively young patients with small-diameter (4 mm or less) unruptured dorsal ICA aneurysms, microsurgery could serve as a good alternative to endovascular treatment.
Considering the anatomical location, microsurgery for dorsal ICA aneurysms is relatively simpler than for conventional paraclinoid aneurysms because total ACP removal is not necessary. Additionally, this approach has the advantage of reducing operation times since the aneurysm can be clipped by merely opening the carotid and optic cistern without dissecting the Sylvian fissure.
Given that dorsal ICA aneurysms occur on the dorsal ICA wall without branches, direct neck clipping can be challenging in cases with a broad neck and surrounding atherosclerotic findings [2]. However, in cases with small size, clipping is relatively easy using 1 or 2 clips. In particular, temporarily clipping a cervical ICA to reduce the pressure on the aneurysm facilitates safer and easier clipping [8].
In this study, 3D CTA with bone image was the most critical preoperative investigation for determining surgical strategies for small unruptured dorsal ICA aneurysms. 3D CTA with bone image allowed us to understand the anatomical relationship between the aneurysm and the ACP. This anatomical relationship significantly aided in deciding whether to remove the ACP in the surgical field. In some cases, 3D CTA with bone image enabled us to predict that aneurysm clipping could be performed without ACP removal and falciform ligament cutting [17]. If imaging showed that the aneurysm was located distal to the ACP, the aneurysm could likely be clipped without ACP removal.
During microsurgery for dorsal ICA aneurysms, exposure of the cervical ICA is deemed essential for proximal control. If the ACP is not fully removed intraoperatively, proximal control in the surgical field is challenging. To secure the necessary space for proximal control, it is safer to achieve it through the cervical ICA rather than additional removal of the ACP. Temporarily clipping the intracranial ICA in tight surgical spaces increases the risk of optic nerve injury and intraoperative rupture. Therefore, it is crucial to expose the cervical ICA to achieve proximal control. In our cases, all aneurysms were clipped under the proximal control of the cervical ICA to reduce aneurysmal pressure and prevent premature rupture. Consequently, there were no premature ruptures before the clipping of the aneurysm [8]. However, it is important to acknowledge certain limitations when performing clipping for small aneurysms, including the need for additional neck incisions and the potential for increased surgical duration.
The most challenging aspect of microsurgery for paraclinoid aneurysms is removal of the ACP. ACP removal can be time-consuming and difficult, even for experienced neurovascular surgeons, and it can sometimes cause complications [6]. However, in many cases in our surgical series, direct clipping was possible without ACP removal. Even when ACP removal was needed, minimal removal of the ACP using a drill was sufficient to allow for safe aneurysm clipping [6]. In this study, 13 of 25 patients did not require ACP removal, 8 of whom were able to be clipped directly without the need to cut the falciform ligament.
We believe it is important to use preoperative 3D CTA with bone image to identify aneurysms that do not require intraoperative ACP removal. If 3D CTA with bone image suggests that clipping is possible without ACP removal, microsurgery is considered a good option. Preoperative 3D CTA with bone image is valuable in this regard, as it clearly shows the relationship between the aneurysm and the ACP.
We performed microsurgery on patients with unruptured dorsal ICA aneurysms with diameters of 4 mm or less, achieving favorable outcomes in all cases. For small, unruptured dorsal ICA aneurysms, microsurgery entailing proximal control of the cervical ICA can be a suitable approach, particularly when 3D CTA with bone image substantiates a clear anatomical association with the ACP. This suggests that microsurgery could serve as a viable alternative for patients with dorsal ICA aneurysms when endovascular treatment options are limited.
Fig. 1.
Schematic diagram illustrating the strategy for clipping a small dorsal ICA aneurysm. A, B: Aneurysm was immediately exposed (A). Direct neck clipping was performed without removing the falciform ligament (B). C, D, E: Aneurysm was partially obscured by the falciform ligament (C). To expose it, the ligament was incised using a sickle knife (D). The aneurysm was sufficiently exposed to allow direct clipping without further partial ACP removal (E). F, G, H, I: Aneurysm was partially obscured by the falciform ligament (F). The aneurysm was not fully exposed after falciform ligament removal (G). After partial ACP removal, the fully exposed aneurysm (H) was clipped successfully (I). ICA, internal carotid artery; ACP, anterior clinoid process; FL, falciform ligament; ON, optic nerve
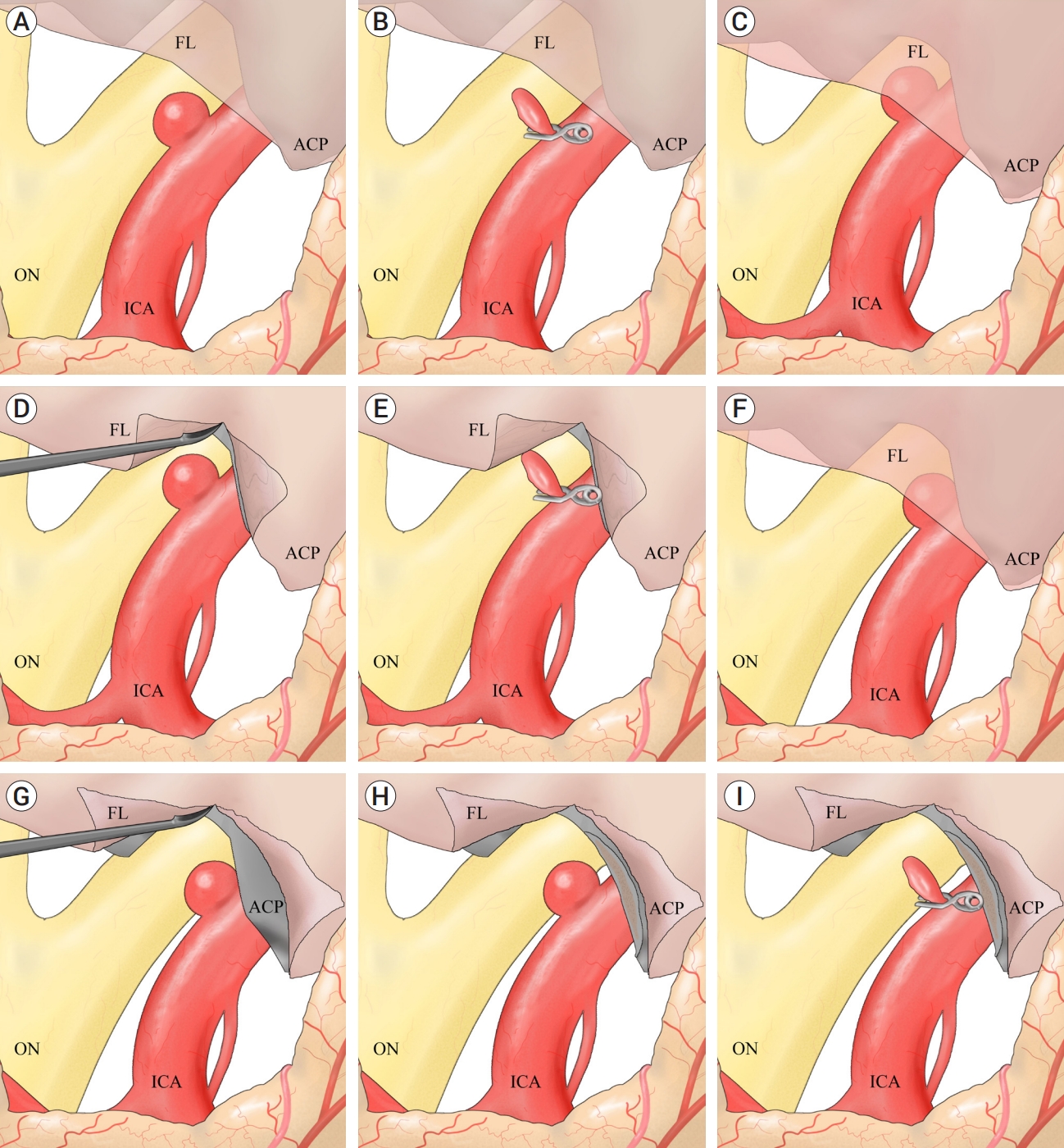
Fig. 2.
Flow chart outlining the surgical strategies. ICA, internal carotid artery; FL, falciform ligament; ACP, anterior clinoid process
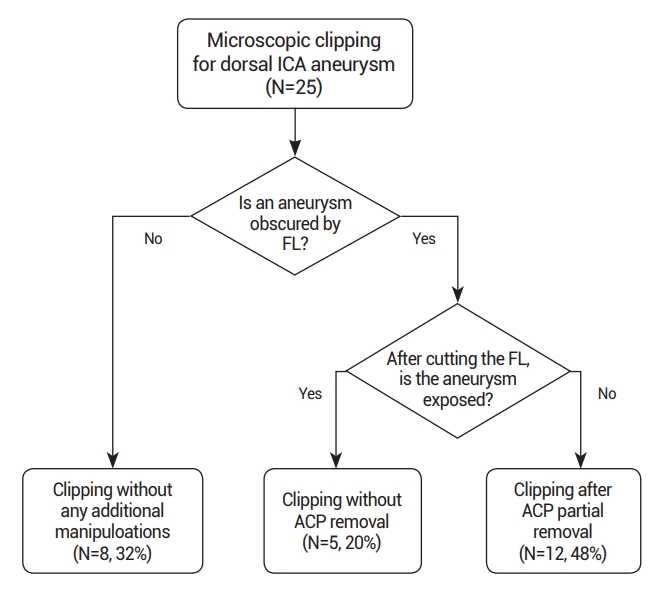
Fig. 3.
Case illustration 1: Aneurysm clipped without the need to cut the falciform ligament. (A) On the 25° rotated digital subtraction angiography image, a dorsal ICA aneurysm is observed distal to the ophthalmic artery. (B) 3D CTA with bone image revealed that the aneurysm (arrow) was situated distally to the ACP. (C) The aneurysm was not obscured by the falciform ligament. (D) The aneurysm was clipped without cutting the falciform ligament. ICA, internal carotid artery; CTA, computed tomography angiography; ACP, anterior clinoid process
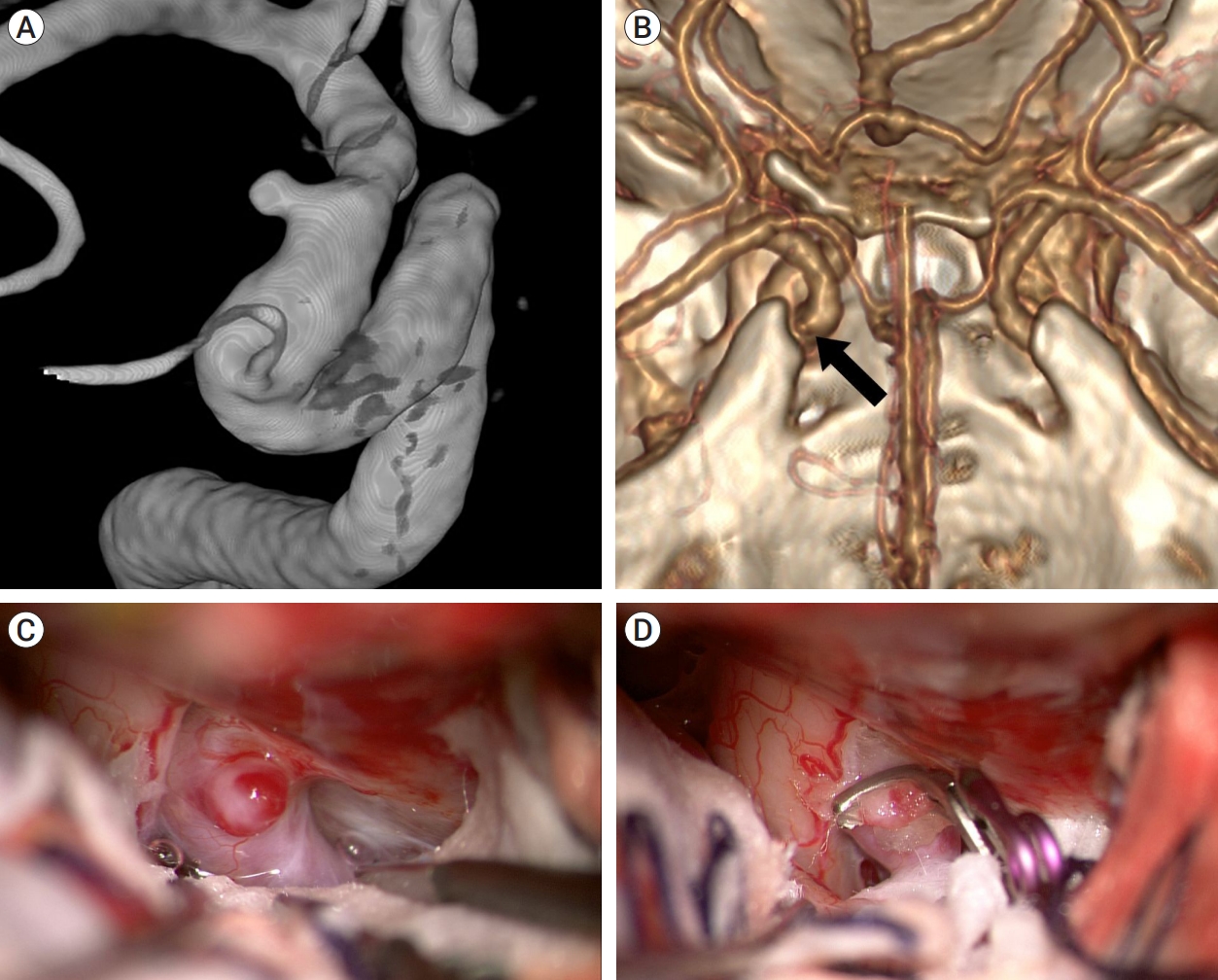
Fig. 4.
Case illustration 2: Aneurysm clipped with only the falciform ligament being cut. (A) 3D CTA with bone image revealed that aneurysm (white arrow) was located distal to ACP. (B) The aneurysm obscured by the falciform ligament. (C) Aneurysm after cutting of the falciform ligament (white arrow). (D) The aneurysm was fully exposed, allowing for successful clipping without the ACP removal. CTA, computed tomography angiography; ACP, anterior clinoid process
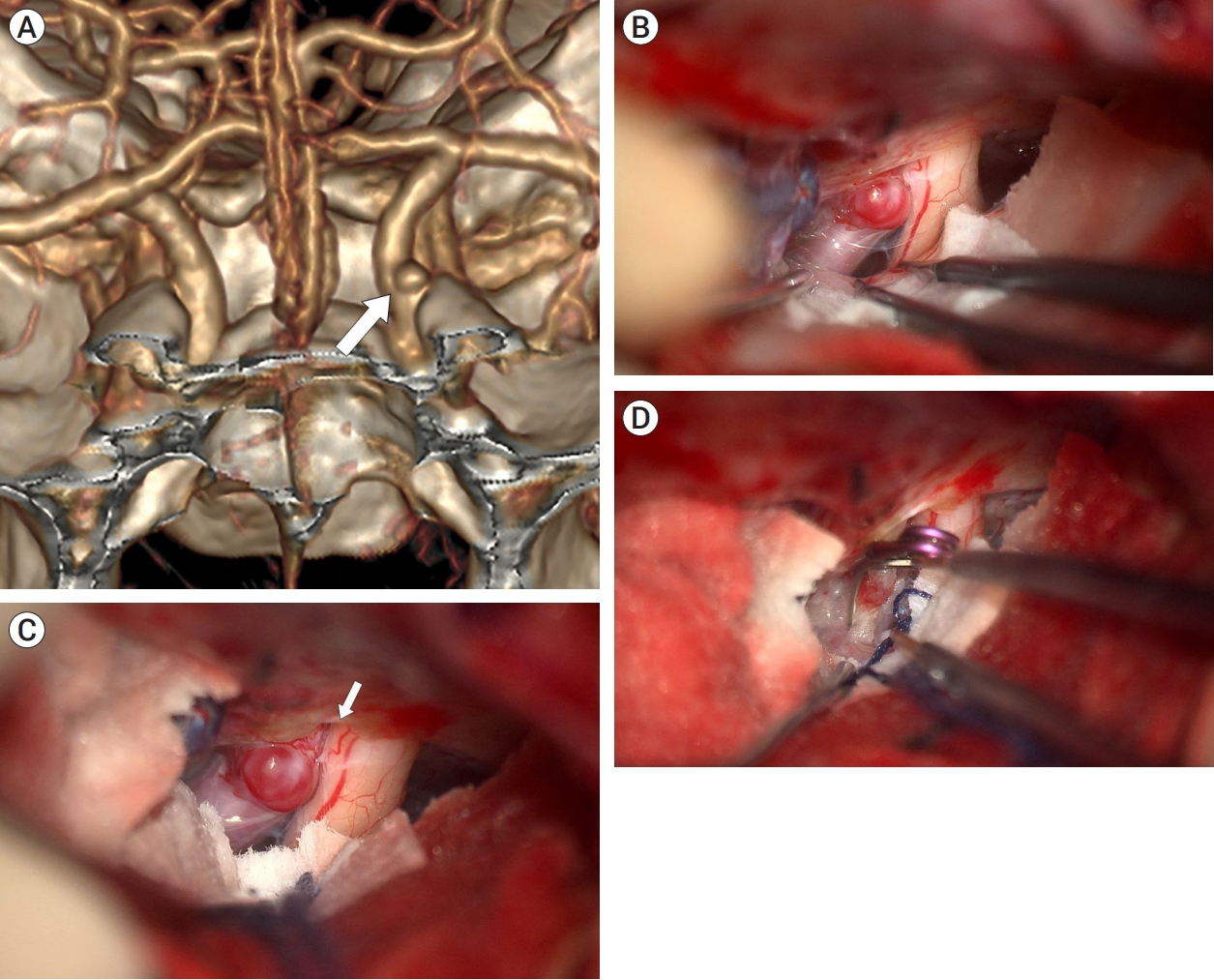
Fig. 5.
Case illustration 3: Aneurysm clipped with both the falciform ligament being cut and partial removal of the ACP. (A) Preoperative digital subtraction angiography revealed an aneurysm with a bleb situated distal to the ophthalmic artery. (B) 3D CTA with bone image showed that the aneurysm (white arrow) was located medial to the ACP, rather than distal to it. (C) The aneurysm was obscured by the falciform ligament. (D) After cutting of the falciform ligament (white arrow), partial removal of the ACP (black arrow) was performed for improved exposure of the aneurysm. (E) The aneurysm was successfully clipped. ACP, anterior clinoid process; CTA, computed tomography angiography
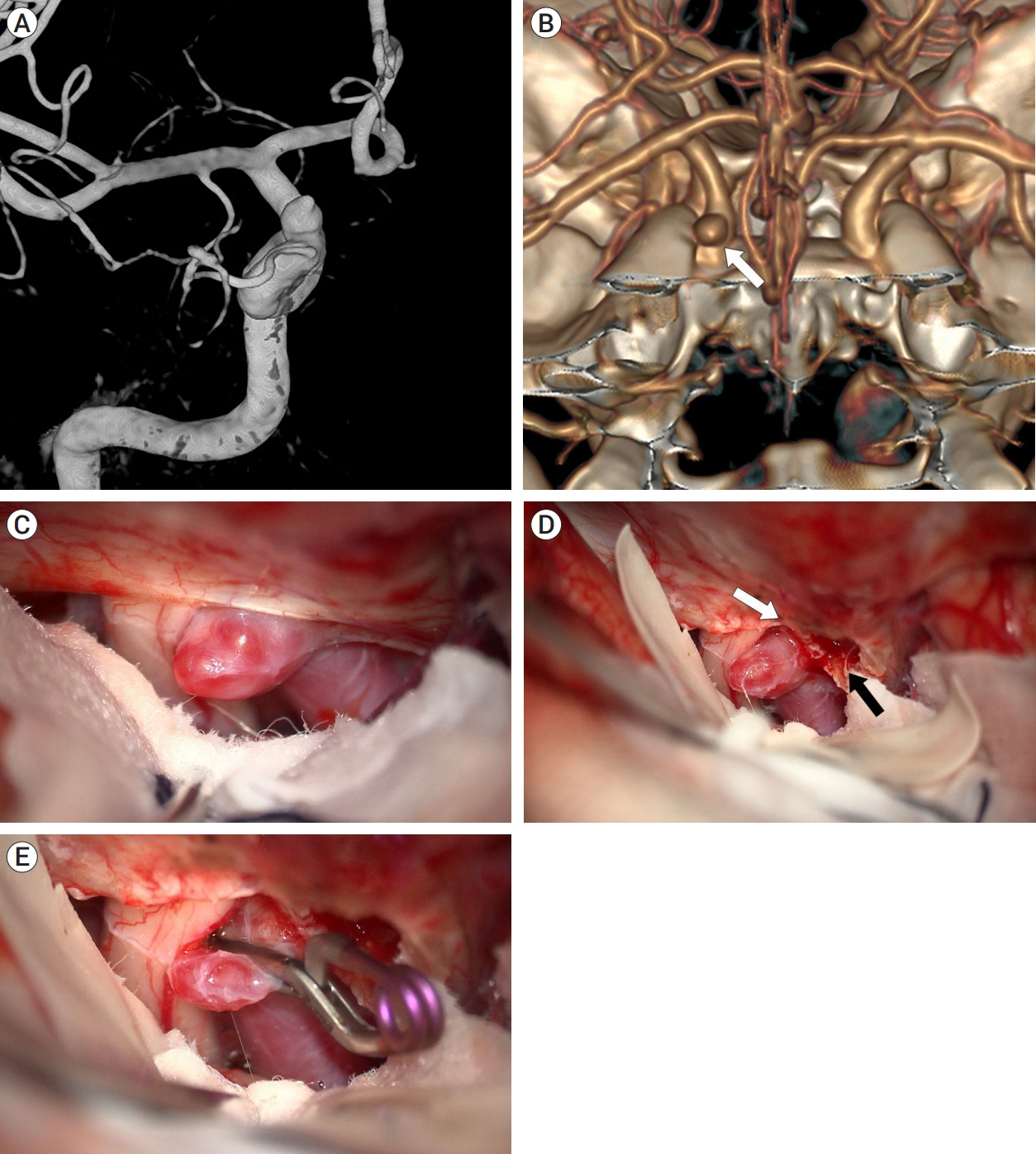
Table 1.
Demographic and clinical data of all patients
REFERENCES
1. Geyik S, Yavuz K, Yurttutan N, Saatci I, Cekirge HS. Stent-assisted coiling in endovascular treatment of 500 consecutive cerebral aneurysms with long-term follow-up. AJNR Am J Neuroradiol. 2013 Nov-Dec;34(11):2157-62.



2. Hong CK, Ahn JY, Joo JY. Internal carotid artery trunk aneurysms-management and outcome. Korean Journal of Cerebrovascular Surgery. 2006 8(2):91-5.
3. Kachhara R, Nair S, Nigam P. Ophthalmic segment aneurysms: Surgical treatment and outcomes. J Neurosci Rural Pract. 2021 Oct;12(4):635-41.



4. Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke. 2000 Oct;31(10):2369-77.


5. Krisht AF, Hsu SP. Paraclinoid aneurysms: Part 1: Superior (true ophthalmic) aneurysms. Contemporary Neurosurgery. 2008 30(15):1-5.
6. Kulwin C, Tubbs RS, Cohen-Gadol AA. Anterior clinoidectomy: Description of an alternative hybrid method and a review of the current techniques with an emphasis on complication avoidance. Surg Neurol Int. 2011 2:140.



7. Kwon BJ, Im SH, Park JC, Cho YD, Kang HS, Kim JE, et al. Shaping and navigating methods of microcatheters for endovascular treatment of paraclinoid aneurysms. Neurosurgery. 2010 Jul;67(1):34-40; discussion 40.



8. Larson JJ, Tew JM Jr, Tomsick TA, van Loveren HR. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: Long-term follow-up of 58 patients. Neurosurgery. 1995 Jan;36(1):26-30; discussion 30.


9. Liu J, Jing L, Wang C, Paliwal N, Wang S, Zhang Y, et al. Effect of hemodynamics on outcome of subtotally occluded paraclinoid aneurysms after stent-assisted coil embolization. J Neurointerv Surg. 2016 Nov;8(11):1140-7.


10. McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, et al. The barrow ruptured aneurysm trial. J Neurosurg. 2012 Jan;116(1):135-44.


11. Mitchell PJ, Muthusamy S, Dowling R, Yan B. Does small aneurysm size predict intraoperative rupture during coiling in ruptured and unruptured aneurysms? J Stroke Cerebrovasc Dis. 2013 Nov;22(8):1298-303.


12. Molyneux A, Kerr R; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group, Stratton I, Sandercock P, Clarke M, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomized trial. J Stroke Cerebrovasc Dis. 2002 Nov-Dec;11(6):304-14.


13. Ries T, Buhk JH, Kucinski T, Goebell E, Grzyska U, Zeumer H, et al. Intravenous administration of acetylsalicylic acid during endovascular treatment of cerebral aneurysms reduces the rate of thromboembolic events. Stroke. 2006 Jul;37(7):1816-21.


14. Ryttlefors M, Enblad P, Kerr RS, Molyneux AJ. International subarachnoid aneurysm trial of neurosurgical clipping versus endovascular coiling: Subgroup analysis of 278 elderly patients. Stroke. 2008 Oct;39(10):2720-6.


15. Shin DS, Carroll CP, Elghareeb M, Hoh BL, Kim BT. The evolution of flow-diverting stents for cerebral aneurysms; Historical review, modern application, complications, and future direction. J Korean Neurosurg Soc. 2020 Mar;63(2):137-52.




- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,126 View
- 34 Download
- ORCID iDs
-
Tae-Sun Kim

https://orcid.org/0000-0001-7134-073X - Related articles
-
Microsurgical Anatomy of the Supraclinoid Internal Carotid Artery.2000 September;2(2)
Strategy & Pitfalls of Internal Carotid Artery Aneurysm Surgery.2003 September;5(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



